Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
4.7 (187) In stock

For values of z near 1, it is a good approximation to write z(P) = 1 + ( z/ P) T P if z = 1.00104  at 298 K and 1 bar, and the Boyle temperature of the gas is 155 K, calculate the values of a, b, and  for the van der Waals gas.  
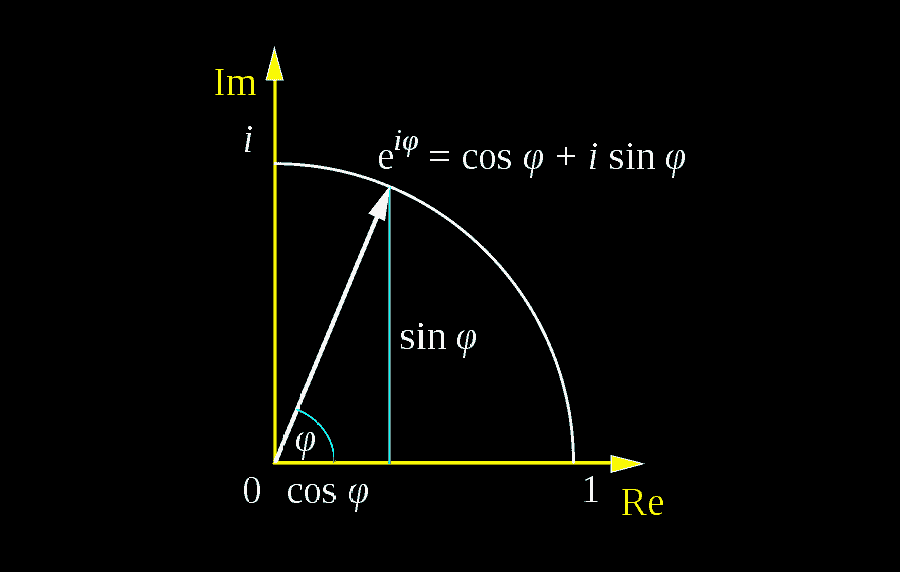
Euler's Formula: A Complete Guide
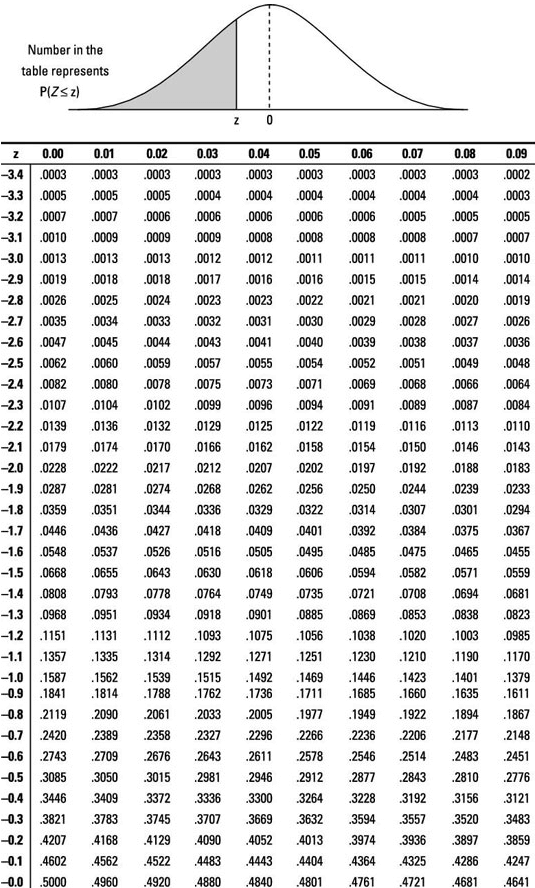
P Value Formula - What Is It, How To Calculate, Examples
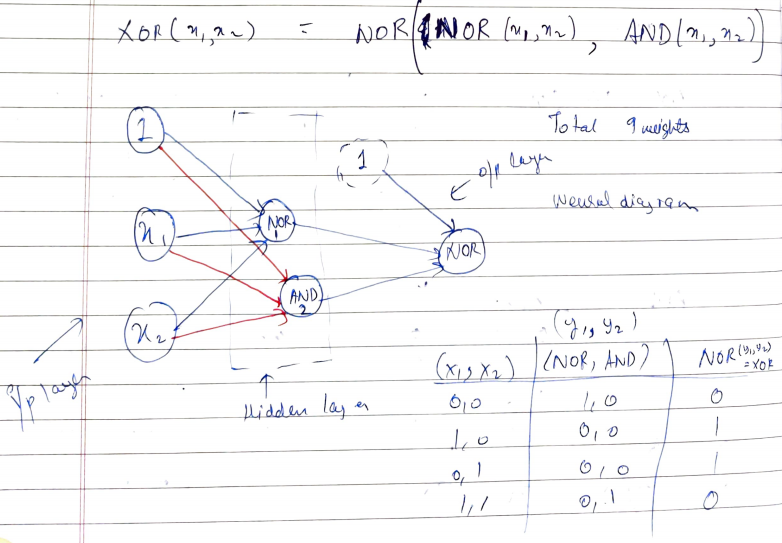
Implementing Logic Gates using Neural Networks (Part 2), by Vedant Kumar
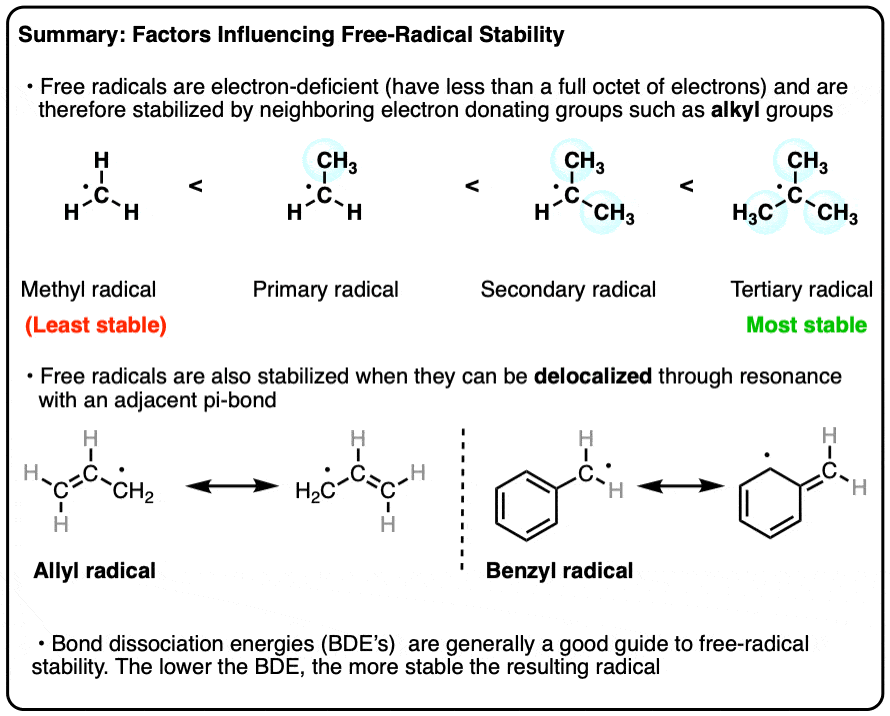
3 Factors That Stabilize Free Radicals – Master Organic Chemistry
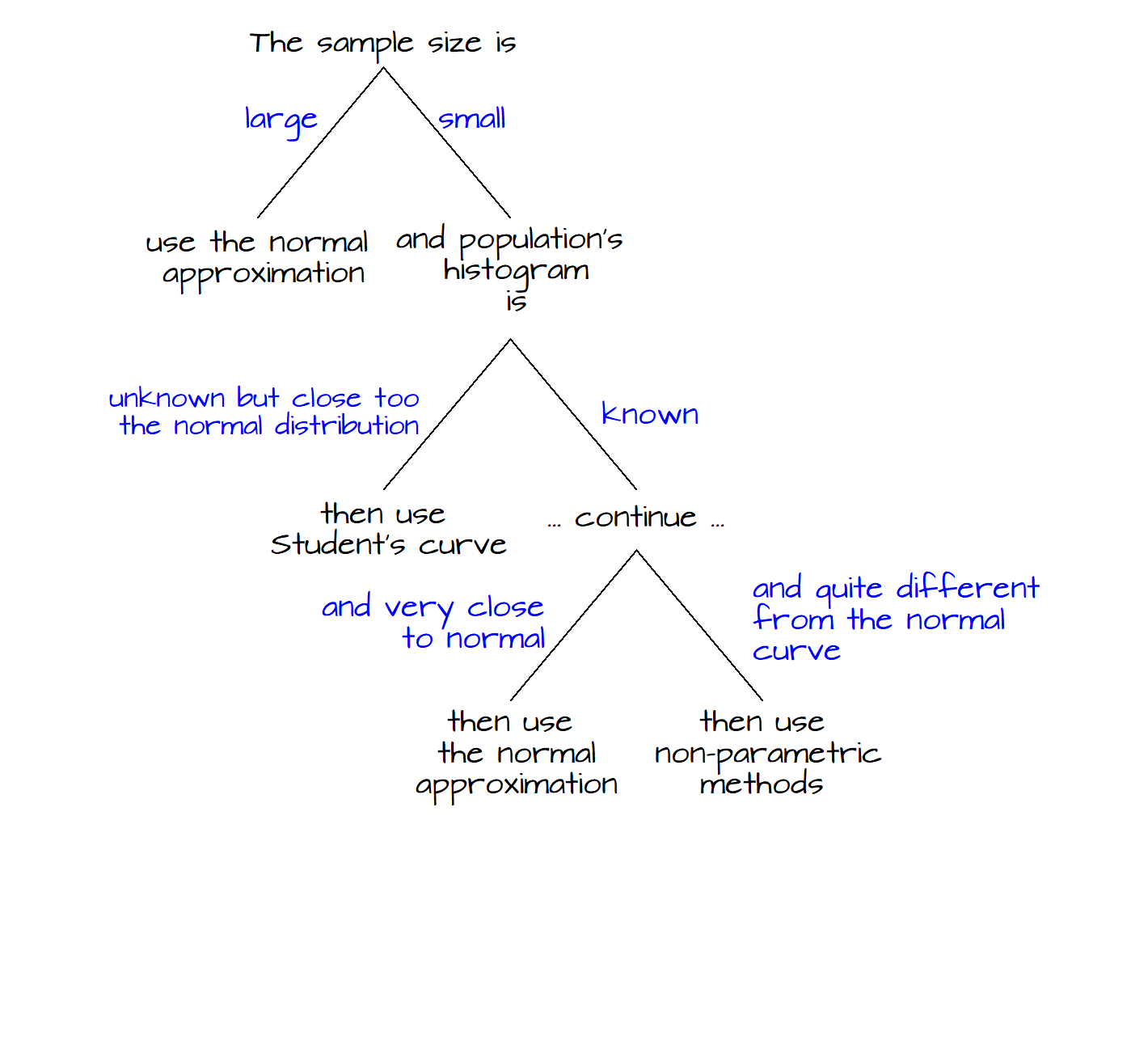
Basic stats explained (in R) - Comparing means: z and t tests

Solved Find the center of mass of the solid cone bounded by
Basic Statistical Analysis Using the R Statistical Package
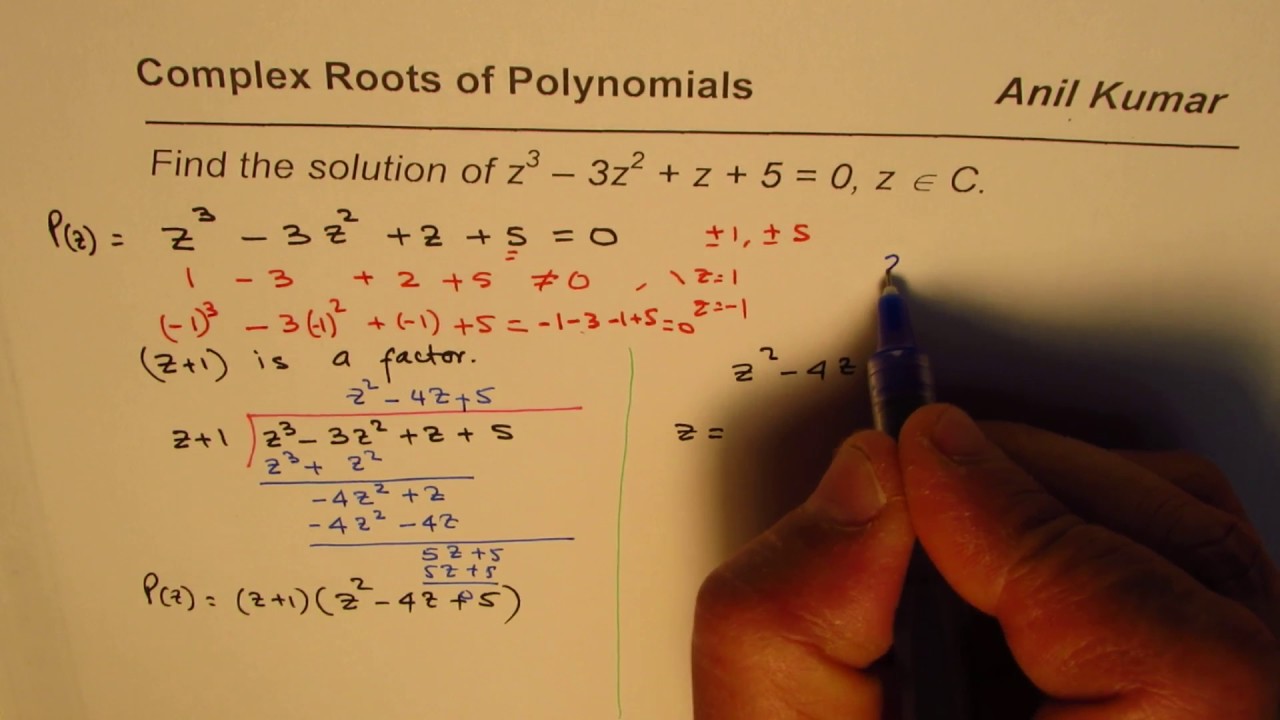
Find Complex Roots of a Cubic Equation z^3 - 3z^2 + z + 5 = 0
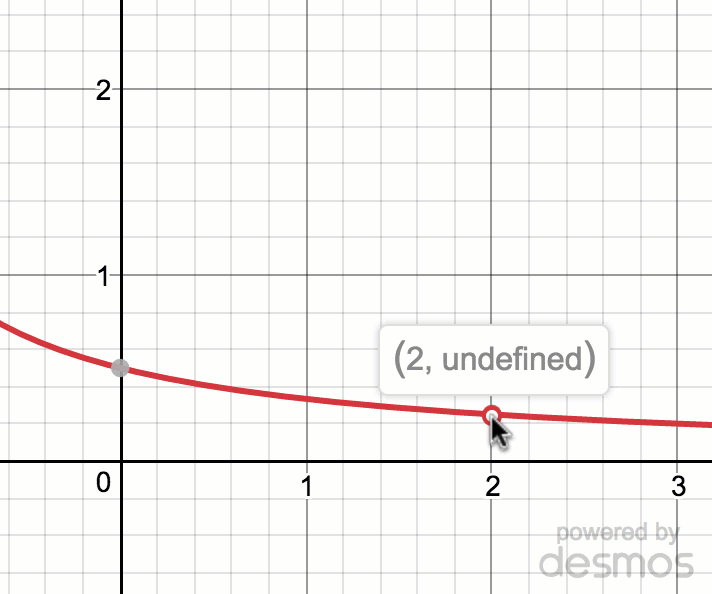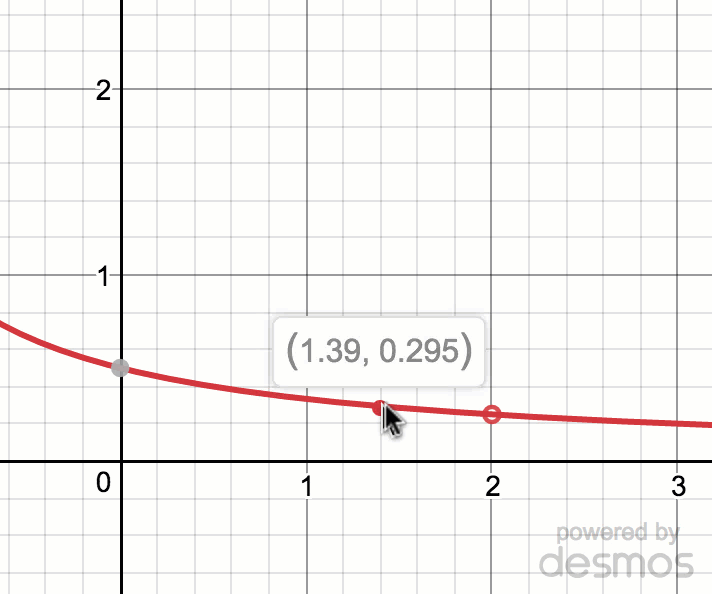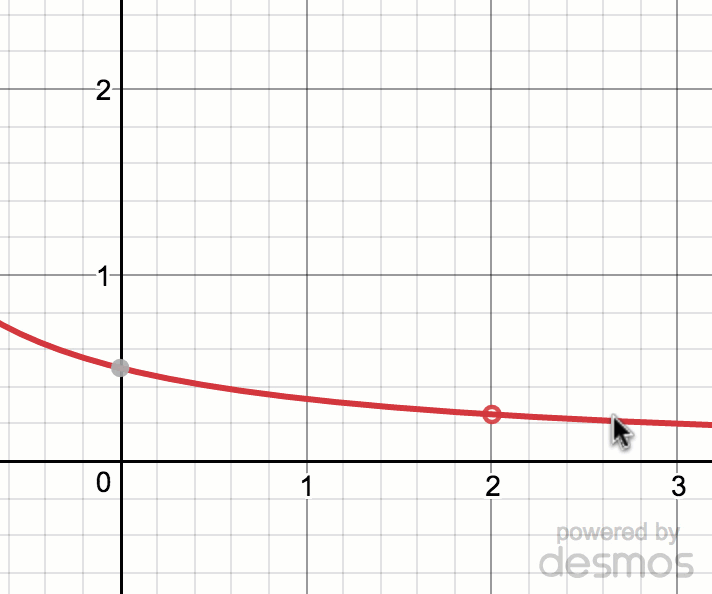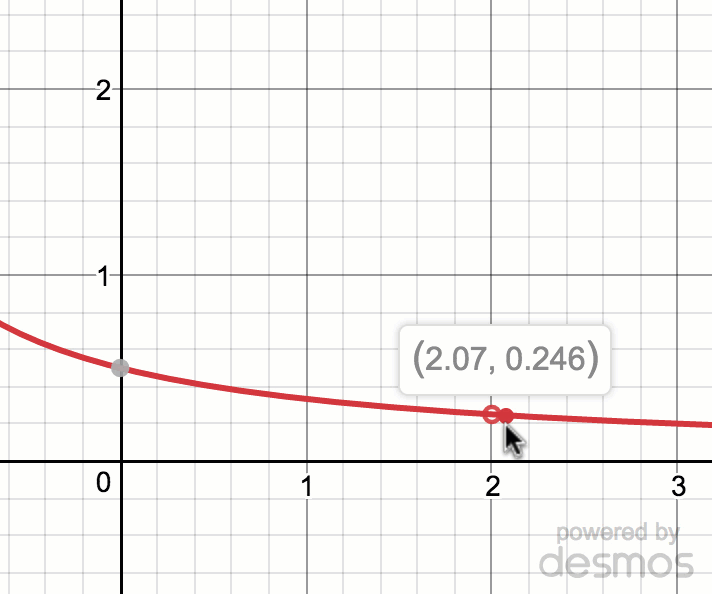
Estimating limit values from graphs (article)
Chapter 8 Real Gases. - ppt download
Solved Z = 4. We saw in class that the compression factor
The compression factor (compressibility factor) for 1 mol of a van der
 This Mom's Instagram About Her Breastfeeding Boobs Is So Relatable
This Mom's Instagram About Her Breastfeeding Boobs Is So Relatable- Thinx For All period proof hiphugger brief with super absorbency in black - BLACK
 Strappy Satin Plunge Criss Cross Supportive Lingerie Bra – lunalae
Strappy Satin Plunge Criss Cross Supportive Lingerie Bra – lunalae Nike Brasilia Extra Small Duffel Bag in Blue
Nike Brasilia Extra Small Duffel Bag in Blue 6 pack No Show thong pack for women thongs for women seamless
6 pack No Show thong pack for women thongs for women seamless Big boob oelan_lory - cracks egg with tits and nip slip
Big boob oelan_lory - cracks egg with tits and nip slip
