Fresenius Kabi Receives FDA 510(k) Clearance for Wireless Agilia Connect Infusion System with Vigilant Software Suite-Vigilant Master Med Technology
5 (600) In stock
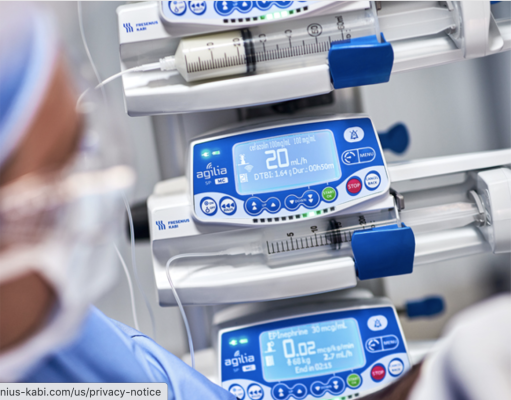
March 22, 2022 – Fresenius Kabi, a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition, has announced it has received 510(k) regulatory clearance from the U.S. Food and Drug Administration (FDA) for its wireless Agilia Connect Infusion System which includes the Agilia Volumetric Pump and the Agilia Syringe Pump with Vigilant Software Suite-Vigilant Master Med technology. The Agilia Connect volumetric pump and syringe pump are the first to be cleared by following TIR101 standards, which were developed by the Association for the Advancement of Medical Instrumentation (AAMI) in 2021. The wireless Agilia Connect Infusion System has been globally available since 2016 and has more than 126,000 customer installations. The Agilia Connect Infusion System builds on the company’s first generation Agilia Infusion System, available in more than 130 countries with more than 1.2 million customer installations, to provide wireless connectivity through the Vigilant Software Suite. This software suite includes Vigilant Master Med drug library software, Vigilant Insight infusion analytics software and Agilia Partner calibration and maintenance software. The product offering enables the centralized distribution of drug libraries, warehousing of infusion data for reporting and analysis and wireless maintenance and calibration of devices. “Both patients and caregivers benefit from smarter devices that make health care safer and easier,” said John Ducker, president and CEO of Fresenius Kabi USA. “As a company with more than 35 years of global infusion technology expertise we look forward to establishing Fresenius Kabi as the partner of choice for infusion therapy in the U.S.” Focused on patient safety and designed to drive best practices, Agilia features customized Master Drug Libraries based on each hospital or clinic’s approved medication protocols. These drug libraries can be distributed wirelessly to an entire fleet of Agilia Connect Pumps, safely and securely. With the Vigilant Insight Continuous Quality Improvement software, infusion data can be configured on demand into reports. This enables providers to conduct independent performance analyses across multiple health care facilities to help reach their infusion practice and patient safety goals. For more information: www.fresenius-kabi.com/us

Fresenius Kabi Receives FDA 510(k) Clearance for Wireless Agilia Connect Infusion System with Vigilant Software Suite-Vigilant Master Med Technology
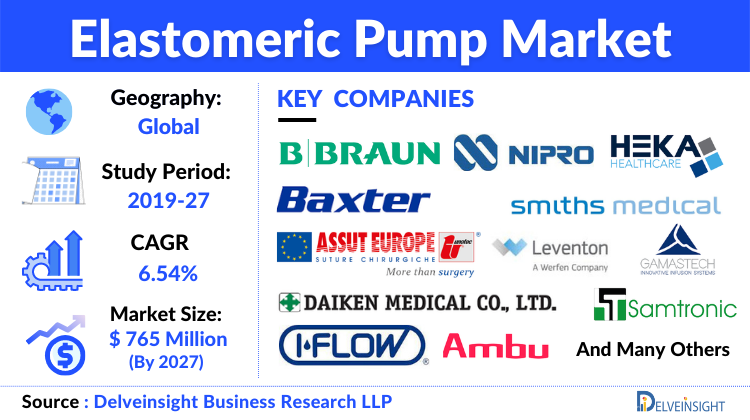
Elastomeric Pump Market to Grow at a CAGR of 6.54% by 2027
Fresenius Kabi Receives U.S. Food and Drug Administration 510(k) Clearance for Wireless Agilia® Connect Infusion System with Vigilant® Software Suite- Vigilant® Master Med Technology

Fresenius Kabi USA LLC M46445660 - McKesson Medical-Surgical

Keith Meadors, MBA, MT(ASCP) on LinkedIn: Fresenius Kabi Receives U.S. Food and Drug Administration 510(k) Clearance…

Fresenius Kabi USA LLC Z021135 - McKesson Medical-Surgical
Pharmaceuticals

Delphine Boulanger on LinkedIn: Fresenius Kabi @ ESICM congress 2022

Food & Drug Administration (FDA) Archives - Page 12 of 85 - Drug Delivery Business

US10245374B2 - Syringe pump - Google Patents
 ASICS Men's Matflex 7 Shoes, 6.5, Black/New Leaf
ASICS Men's Matflex 7 Shoes, 6.5, Black/New Leaf HSMQHJWE Womens Slides Sandals Flat Slide Beach Sandals Ladies Open Toe Rhinestones Bow Beach Slides Summer Slip On Slides Shoes For Women Flat Slide Sandals HSMQHJWE(Multicolor,8)
HSMQHJWE Womens Slides Sandals Flat Slide Beach Sandals Ladies Open Toe Rhinestones Bow Beach Slides Summer Slip On Slides Shoes For Women Flat Slide Sandals HSMQHJWE(Multicolor,8) Bonds Women's Retro Rib Wirefree Bra - Pink - Size 12
Bonds Women's Retro Rib Wirefree Bra - Pink - Size 12 Women's Simple Body Sculpting Adjustable Strap Top Built In Bra
Women's Simple Body Sculpting Adjustable Strap Top Built In Bra How to make the best armor in minecraft 1.20 - You've been doing it WRONG!
How to make the best armor in minecraft 1.20 - You've been doing it WRONG! Wholesale Women's clothing & apparel - Faire Canada
Wholesale Women's clothing & apparel - Faire Canada