Applications for Medical Device Investigational Testing Authorizations Guidance Document
4.8 (237) In stock

Applications for Medical Device Investigational Testing Authorizations Guidance Document

Medical Device Guidelines and Regulations Handbook

Clinical Trial Applications in eCTD Format - Eng - March 2020, PDF, Clinical Trial

Reporting Processes to Health Canada - ppt download

G.500 - PHS Human Subjects and Clinical Trials Information

Clinical Research with Medical Devices 101

validation and verification of medical device.pptx

Medical Device Classification Product Codes - Guidance for Industry and Food and Drug Administration Staff

MHRA Guidance on IDAP Pilot: Application in Detail
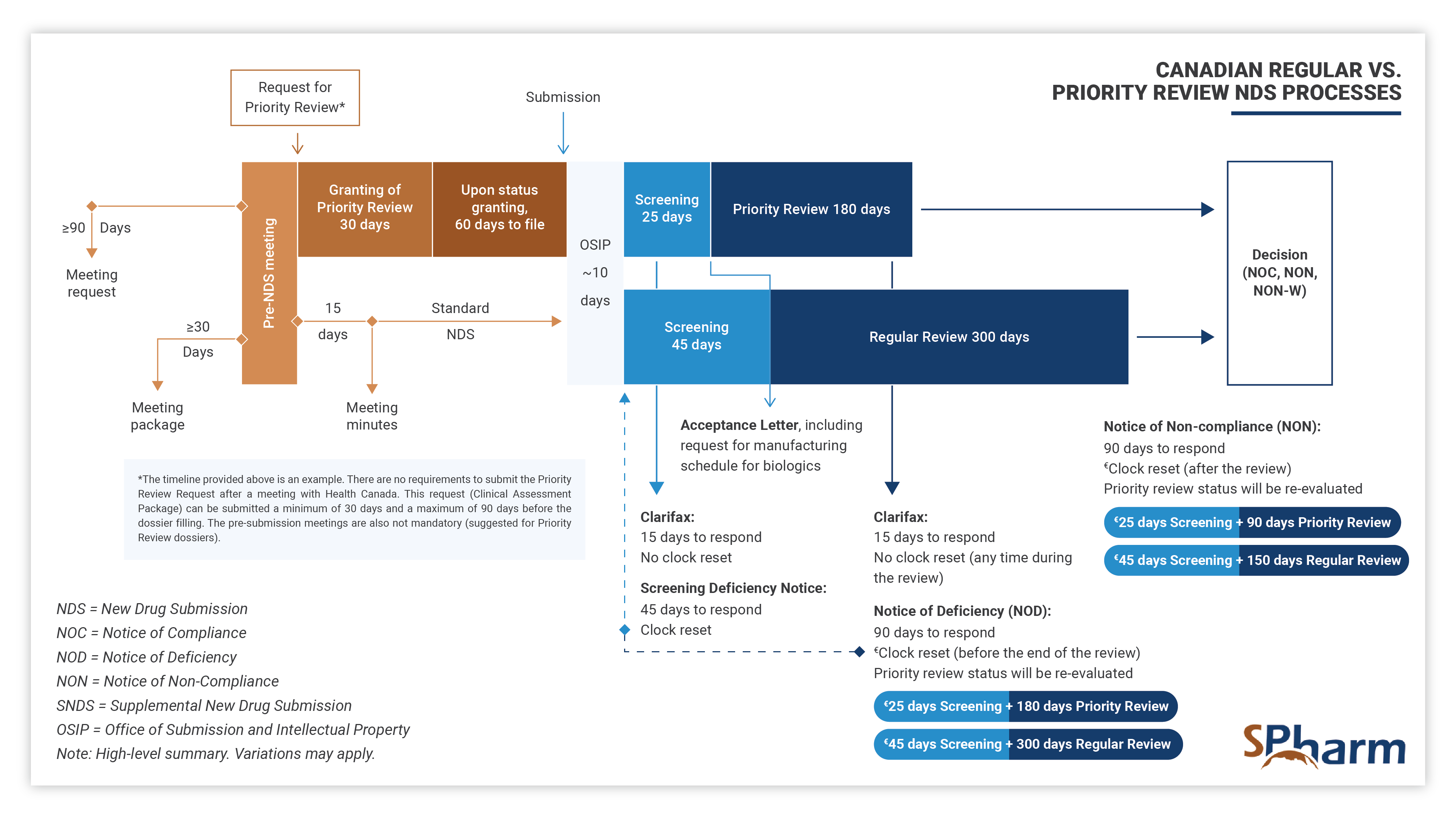
New Drug Submission Process in Canada
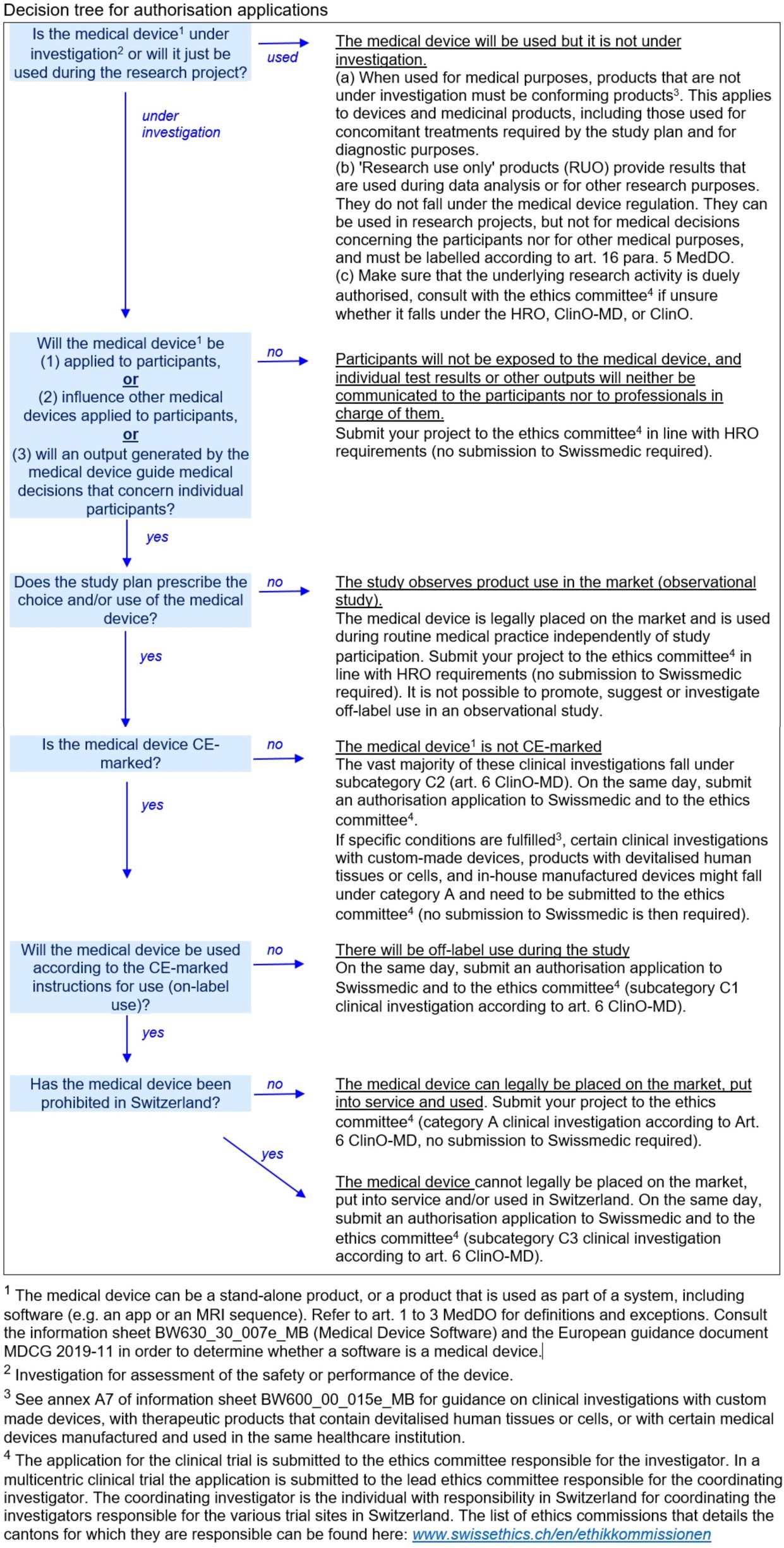
Clinical investigations

Medical device submissions: Placing a medical device on the market
ITA-MED Hernia Support - Neb Medical
ITA-MED - High-Quality, Medical Support Products For A Healthy Lifestyle - SuperbCrew
Clínica Itamed – Clínica de Análises Clínicas
ITA-MED Posture Corrector TLSO-250 review – A complete unbiased
 36c body shaper one - Gem
36c body shaper one - Gem:max_bytes(150000):strip_icc()/27258_DecoratingWithPalms1885-da96a307581e464abab30c973cb24801.jpg) 54 Easy Spring Flower Arrangements You Can Totally Pull Off
54 Easy Spring Flower Arrangements You Can Totally Pull Off Set of 2 Large Metal Frame Wall Sconce, Decorative Wall Candle Sconces, Wall Decor with Candle Holder (Gold)
Set of 2 Large Metal Frame Wall Sconce, Decorative Wall Candle Sconces, Wall Decor with Candle Holder (Gold) Vector Relaxed Pant, Baggy Bottoms, Flat Sketch, for Adobe
Vector Relaxed Pant, Baggy Bottoms, Flat Sketch, for Adobe Loose Khaki Cargo Pants Khaki cargo pants, Cargo pants, Trousers women
Loose Khaki Cargo Pants Khaki cargo pants, Cargo pants, Trousers women The Desnudas are topless women wearing only panties who paint their exposed bodies with body paint and stand in Times Square to pose with tourists fo Stock Photo - Alamy
The Desnudas are topless women wearing only panties who paint their exposed bodies with body paint and stand in Times Square to pose with tourists fo Stock Photo - Alamy