At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry
4.6 (258) In stock

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior. Their equation of state is given as P=RTV−b at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given as P=RTV b at T.Here, b is

In the given figure an ideal gas changes its state from `A` to state `C` by two paths `ABC` and

5-4: Derivation of the Ideal Gas Law An ideal gas is
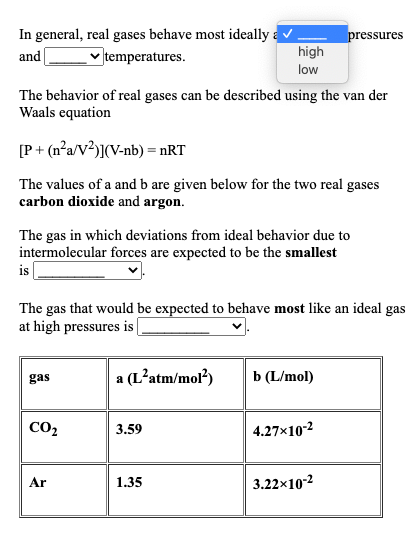
Solved In general, real gases behave most ideally a

Competition Science Vision - February 2008, PDF

At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour.

PDF) Thermal energy storage Diego Armando Gutierrez Diaz
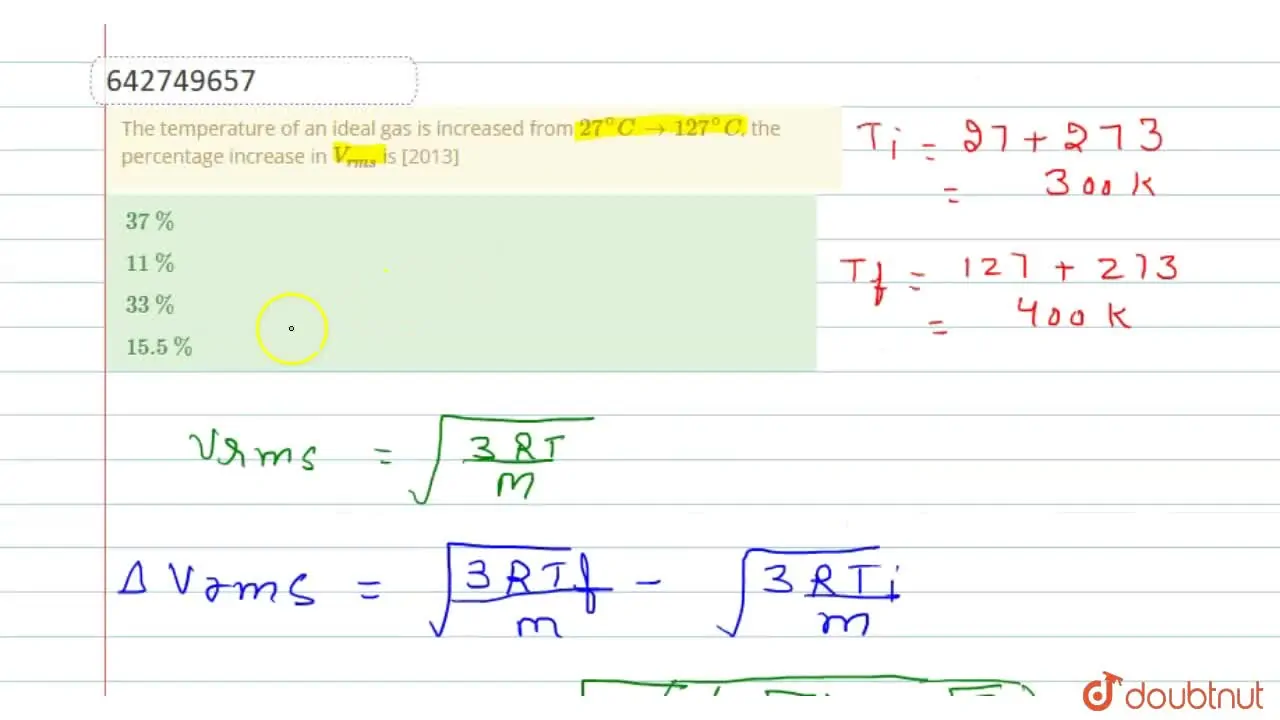
The temperature of an ideal gas is increased from 27^(@)C to 127^(@)C
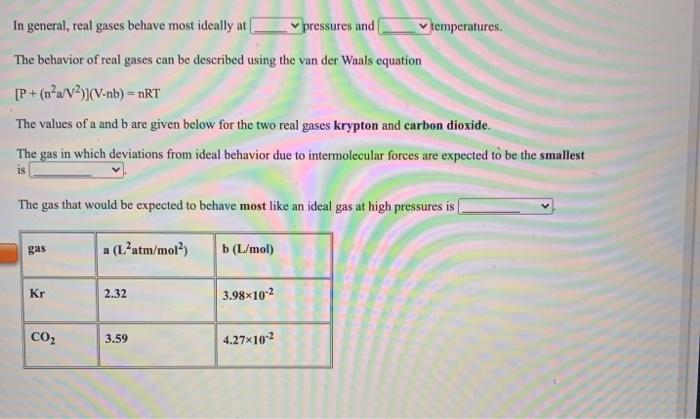
Solved In general, real gases behave most ideally at
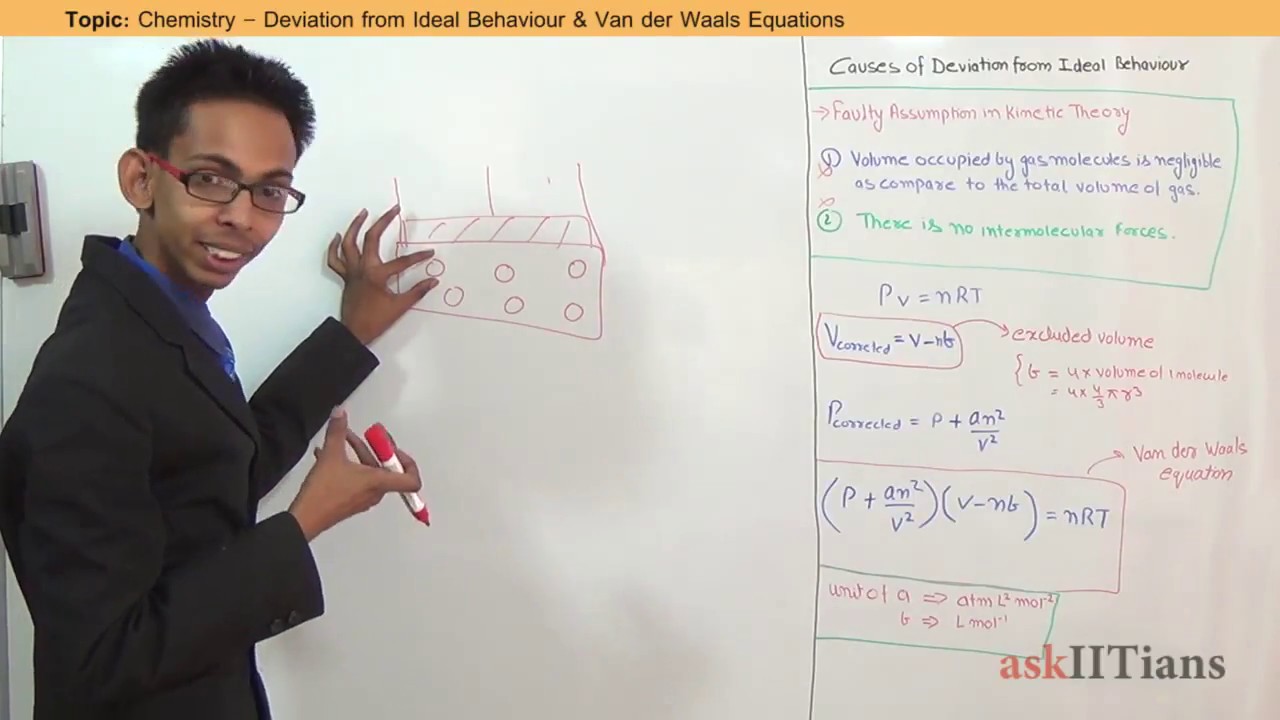
Deviation from Ideal Behavior & Van der Waals Eqn, Chemistry, 11th, IITJEE Main/Adv., NEET
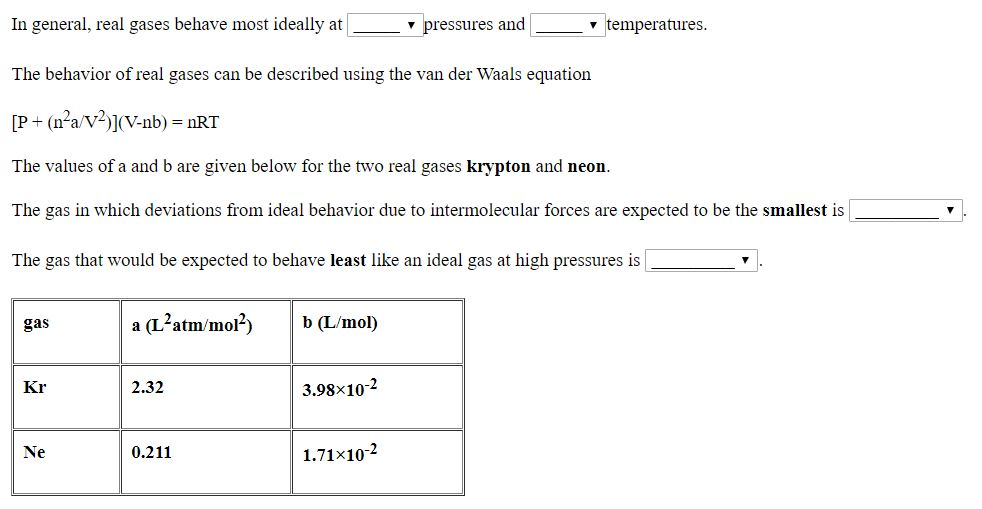
Solved In general, real gases behave most ideally at

PDF) IOSR Journal of Applied Physics (IOSR-JAP)

At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior. jee
Solved 9 Compression factor Z Use the van-der-Waals equation
Answered: Compression factor of a gas with van…
Show that the van der Waals equation leads to values of Z <
What is the value of compression factor Z for the gas? (A) 1 (B) >1 (C) <1 (D) Zero
- Mesh Corset Design Cupped High Leg Bodysuit
 MENS SISSY FRILLY Satin Panties Ruffled Briefs French Maid
MENS SISSY FRILLY Satin Panties Ruffled Briefs French Maid Warners Women's Cloud 9 Wire Free Lift Bra : : Clothing
Warners Women's Cloud 9 Wire Free Lift Bra : : Clothing Victoria's Secret VTG 99' Women's Size 34D Floral Lace Trim Bra
Victoria's Secret VTG 99' Women's Size 34D Floral Lace Trim Bra outstar 🧛🏻 professional vampire simp on X: babe what's wrong
outstar 🧛🏻 professional vampire simp on X: babe what's wrong Scentsationals Festival Vibes Scented Wax Cubes, 2.5 OZ Package : Home & Kitchen
Scentsationals Festival Vibes Scented Wax Cubes, 2.5 OZ Package : Home & Kitchen
