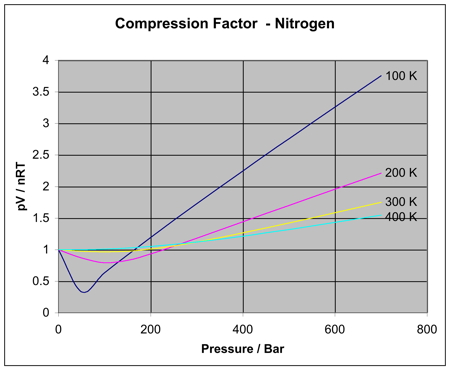
Compression Factor Z
4.5 (688) In stock

Non-ideal behavior of gases (article)

Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect

Compressibility factor - Wikipedia

Real Gas Behavior The Compression Factor (Z) [Example #2]

Compressibility factor Z as function of temperature T with lines

2 Generalized compressibility chart for Z = 0.270

Compressibility factor (Z) for a van der Waals real gas at

e Compressibility factor (Z) for hydrogen WRT pressure and

COMPRESSIBILITY FACTOR
Compression Factor Exam Problem using Molar Volumes - Fully Explained!
Solved) - For values of z near 1, it is a good approximation to
/product/00/7631721/1.jpg?9988) Generic 5.5Yard Face Lift Tape Thin Lifting Sticker Patches For Hide Face @ Best Price Online
Generic 5.5Yard Face Lift Tape Thin Lifting Sticker Patches For Hide Face @ Best Price Online rygai Lady Panties Low Waist Seamless Close-fitting Women Underpants for Daily Wear,Black 3XL
rygai Lady Panties Low Waist Seamless Close-fitting Women Underpants for Daily Wear,Black 3XL Shapewear for Women Tummy Control Yoga Jumpsuits Workout Ribbed
Shapewear for Women Tummy Control Yoga Jumpsuits Workout Ribbed Ardene Man Cargo Jogger Pants For Men in Dark Green
Ardene Man Cargo Jogger Pants For Men in Dark Green Maternity 3/4 Sleeve Sexy Dresses for Women for sale
Maternity 3/4 Sleeve Sexy Dresses for Women for sale/spree/images/attachments/007/523/189/original/wacoal-embrace-lace-black-soft-cup-bra-32-harvey-nichols-photo.jpg) Wacoal Embrace Lace Black Soft-cup Bra, 32
Wacoal Embrace Lace Black Soft-cup Bra, 32