Physical Chemistry The Compression Factor (Z) [w/1 example]
4.9 (502) In stock

Publications from Research Conducted at NOMAD

Compressibility factor for real gases

Physical Chemistry The Compression Factor (Z) [w/1 example]
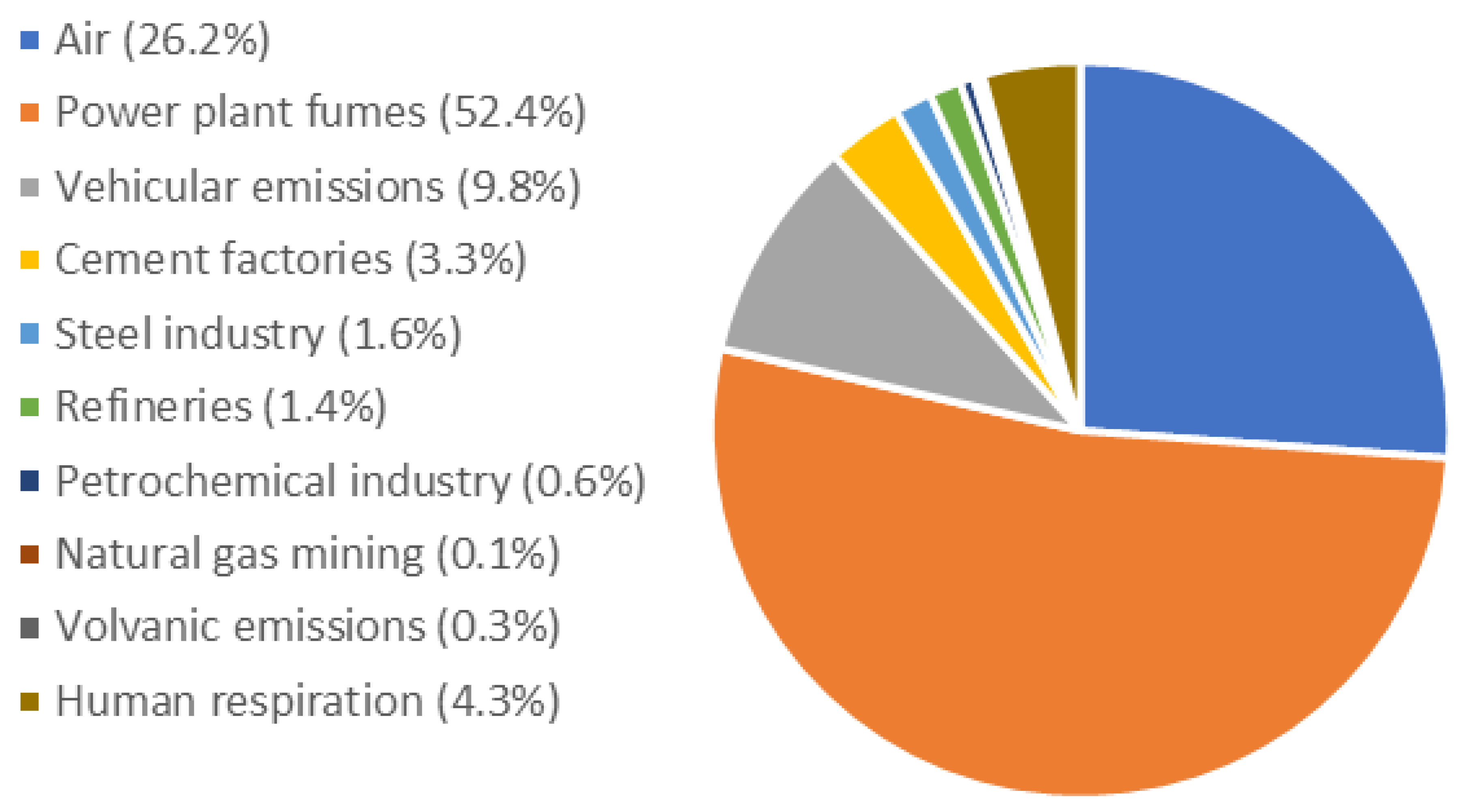
Atmosphere, Free Full-Text

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Solved The compression factor, Z, can also be calculated

6.3: Van der Waals and Other Gases - Physics LibreTexts

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Real gas 1.molecules not always in motion (condense phase can be formed) 2.molecular size is non-negligible (there is molecular repulsion) 3.Molecules. - ppt download

The compression factor compressibility factor for 1 mole of a van der Waals' gas at 0∘ C and 100 atmospheric pressure is found to be 0.5 . Assuming that the volume of

6.3: Van der Waals and Other Gases - Physics LibreTexts

COMPRESSIBILITY FACTOR
Compressibility factor Z = PV / nRT is plotted against pressure as
Compressibility Factor, z vs Pressure, P (kPa), line chart made by Jdvani
 Popcorn Wire-free Nursing Sports Bra
Popcorn Wire-free Nursing Sports Bra Am I a Candidate for a Tummy Tuck? - Modern Surgical Arts of Denver
Am I a Candidate for a Tummy Tuck? - Modern Surgical Arts of Denver Depend FIT-FLEX Incontinence Underwear for Men, Maximum Absorbency, Disposable, S/M, Grey, (Packaging May Vary), 30 Count (Pack of 3) : Health & Household
Depend FIT-FLEX Incontinence Underwear for Men, Maximum Absorbency, Disposable, S/M, Grey, (Packaging May Vary), 30 Count (Pack of 3) : Health & Household Brass Hook Rabbit – Fern
Brass Hook Rabbit – Fern Comfortable Stylish sexy girls braces Deals
Comfortable Stylish sexy girls braces Deals Womens Silicone Strapless Bra Night Bras Women UK 2023 Navy Blue Lace Bandeau Maternity Bras Pregnancy Long Line Bras Women Womens Sleep Bra Strapless
Womens Silicone Strapless Bra Night Bras Women UK 2023 Navy Blue Lace Bandeau Maternity Bras Pregnancy Long Line Bras Women Womens Sleep Bra Strapless