At 300 K, 36 g of glucose present per litre in its solution has an osm
5 (663) In stock

pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

please explain the question and tell me what is 4 98 bar in this question and why it's not been - Chemistry - Solutions - 14451181

At `300 K`, `36 g` of glucose present per litre in its solution has an osmotic pressure of `4.98

Nutrients, Free Full-Text
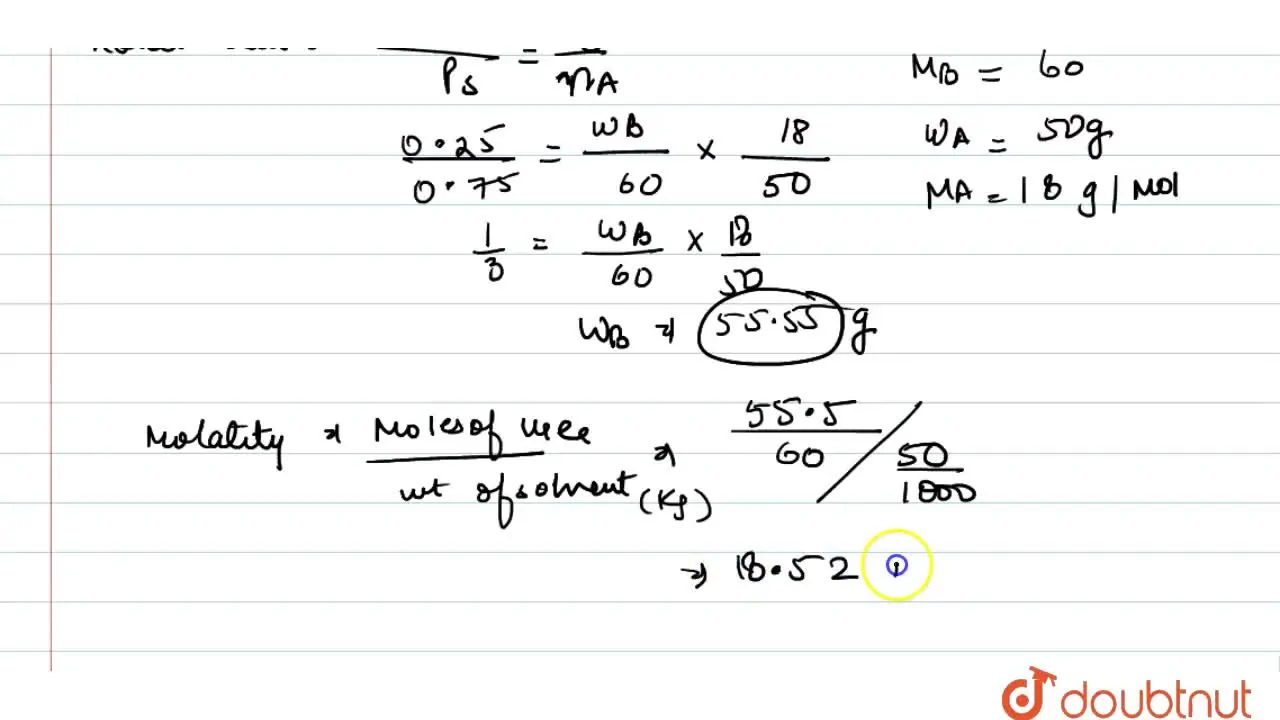
How mich urea (molar mass=60 g mol^(-1)) must be dissolved in 50 g o

2.22At300 K,36 g of glucose present in a litre of its solution has an osm..

Kannada] At 300 K, 36 g of glucose present per litre in its solution

A solution prepared by dissolving 8.95 mg of a gene fragment in 35.0 m
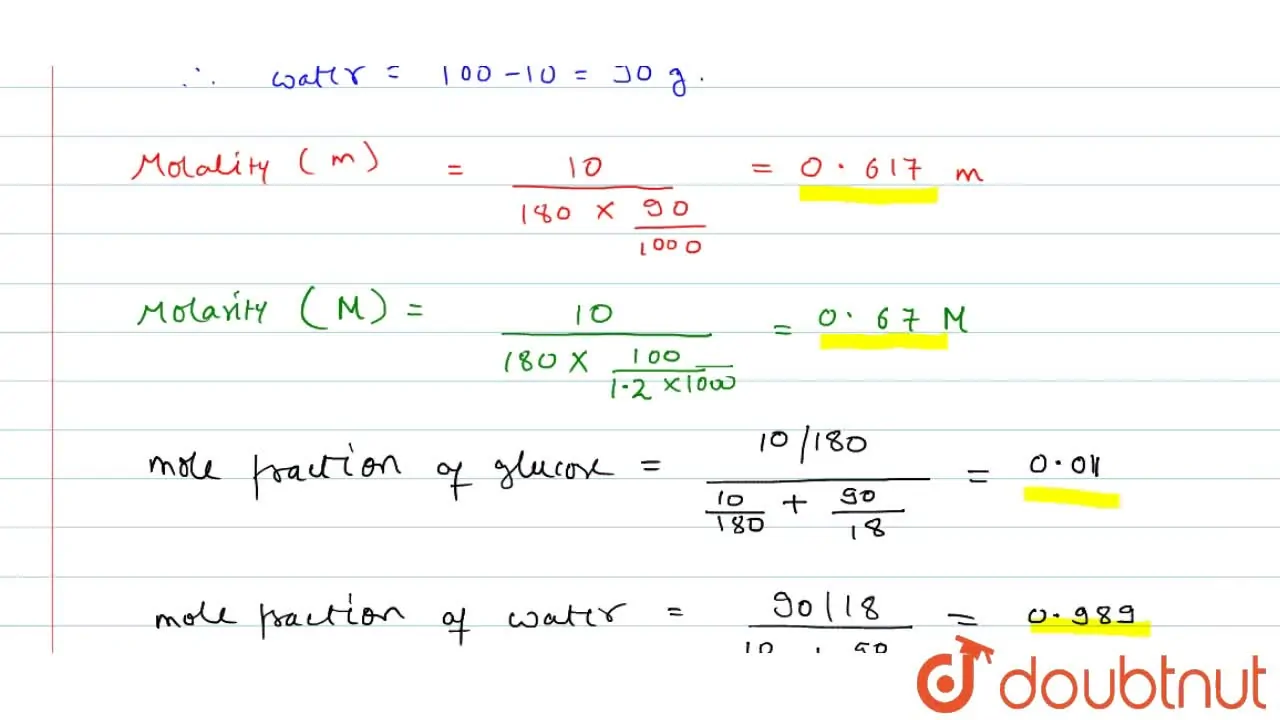
A solution of glucose in water is labelled as 10 percent w//w, what

The osmotic pressure of blood is 8.21 atm at 37^(@)C. How much glucose

At `300K,36g` of glucose present per litre in its solution had an osmotic pressure `4.98 ` bar. If
36 Gramas em Quilogramas conversor de unidades
GEL PARA EXTENSÃO DE UNHA FIBRA LOVE YES 36 G
 Buy digital version: Bust of a young woman with turned 3/4 full head by John Singer Sargent, Boston
Buy digital version: Bust of a young woman with turned 3/4 full head by John Singer Sargent, Boston Transparent Labs Whey Protein Review (2024 Update)
Transparent Labs Whey Protein Review (2024 Update) Solstice SunSoft Red Fabric Island Pool Float
Solstice SunSoft Red Fabric Island Pool Float The Right Way to Speak Truth to Power
The Right Way to Speak Truth to Power How M&S plans to become 'more relevant, more often
How M&S plans to become 'more relevant, more often MEETWEE Womens Thermal Underwear Set, Winter Compression Long
MEETWEE Womens Thermal Underwear Set, Winter Compression Long