At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{
4.8 (308) In stock


Answered: Determine the number of atoms contained…
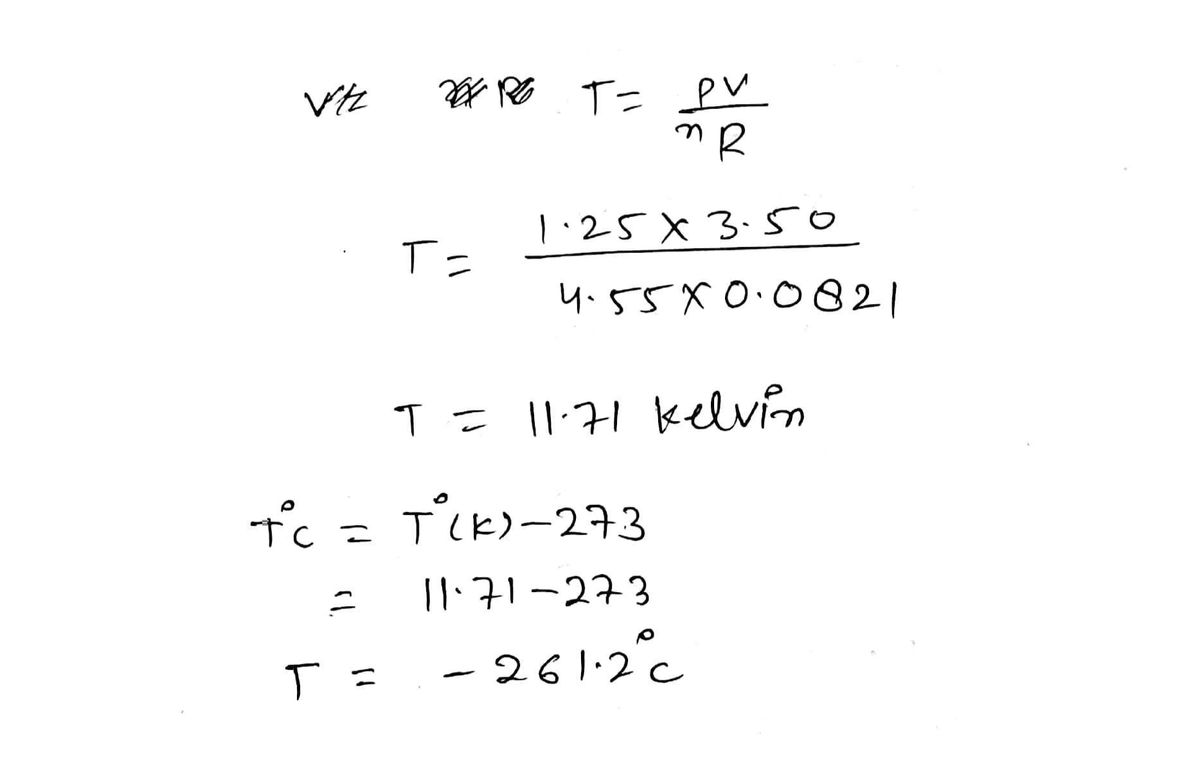
Answered: What is the temperature, in degrees…
Kinetic modelling of rarefied gas flows with radiation, Journal of Fluid Mechanics

The Ideal Gas Law

Dalton's law of partial pressure (article)

Solved 6. Take the van der Waals equation RT a 2 a. Express

Random, PDF, Gases
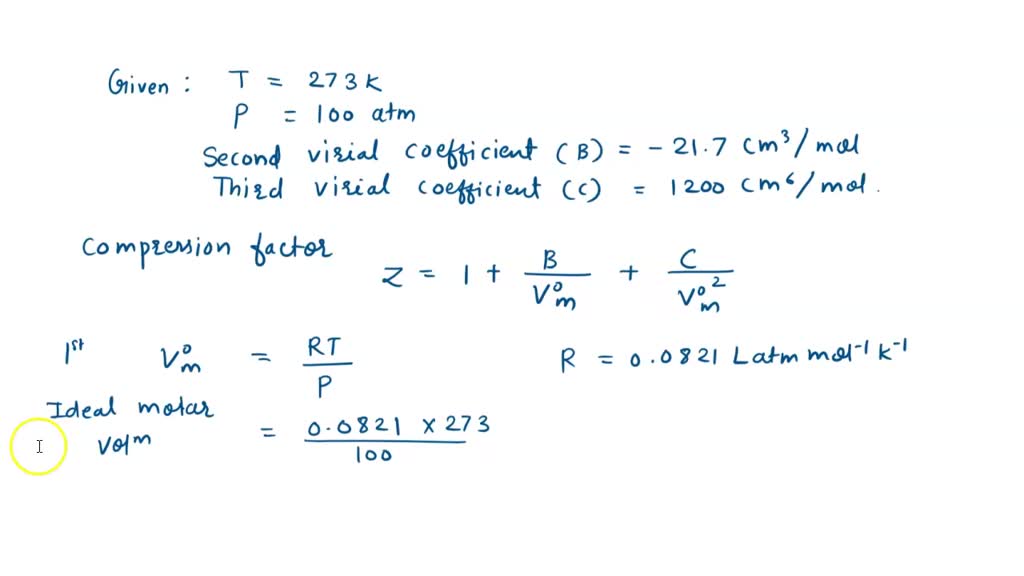
SOLVED: At 273 K, measurements on argon gave B = -21.7 cm^3/mol and C = 1200 cm^6/mol^2, where B and C are the second and third virial coefficients in the expression of

Advanced carbon molecular sieve membranes derived from molecularly engineered cross-linkable copolyimide for gas separations
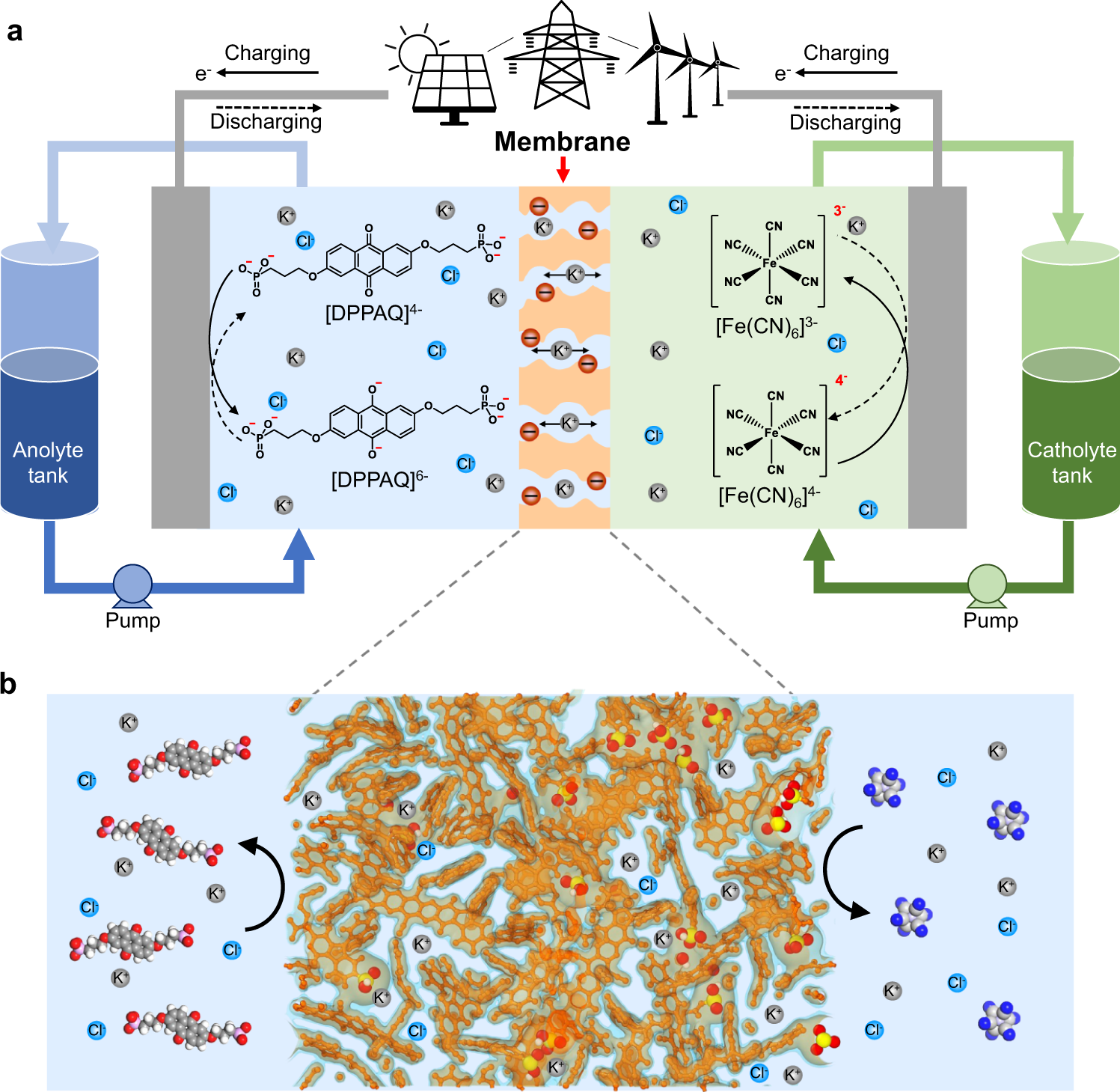
Development of efficient aqueous organic redox flow batteries using ion-sieving sulfonated polymer membranes
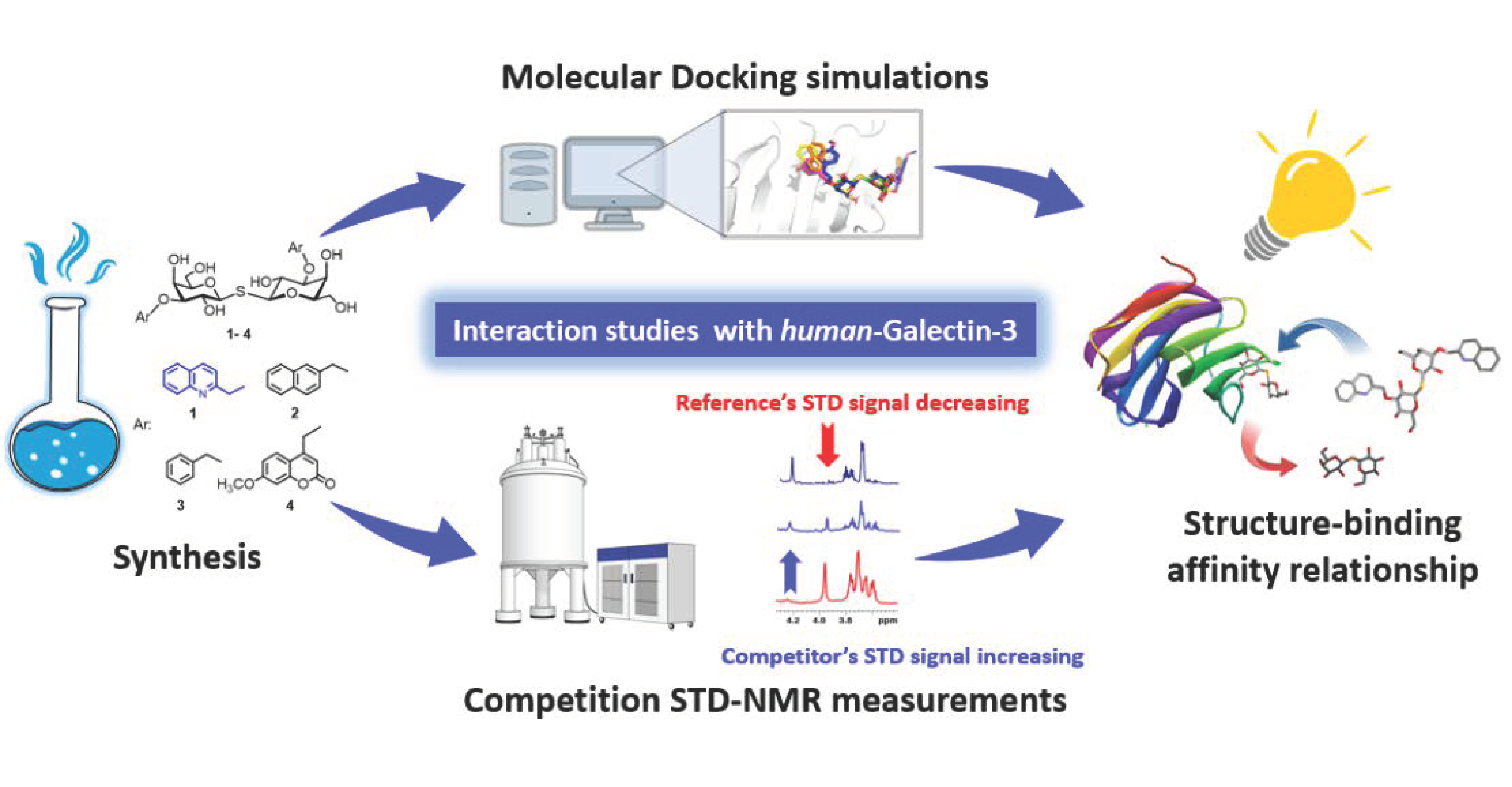
IJMS, Free Full-Text

The apparatus shown consists of three temperature-jacketed 1.000
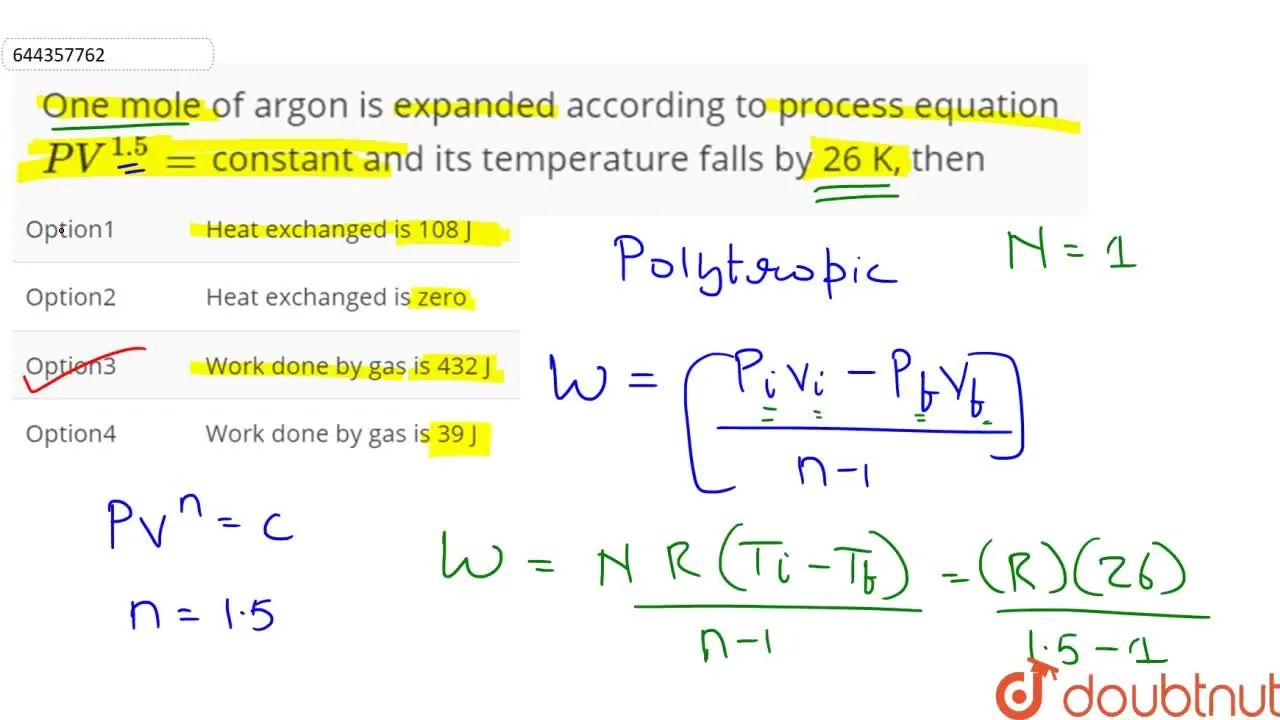
One mole of argon is expanded according to process equation PV^(1.5)=c
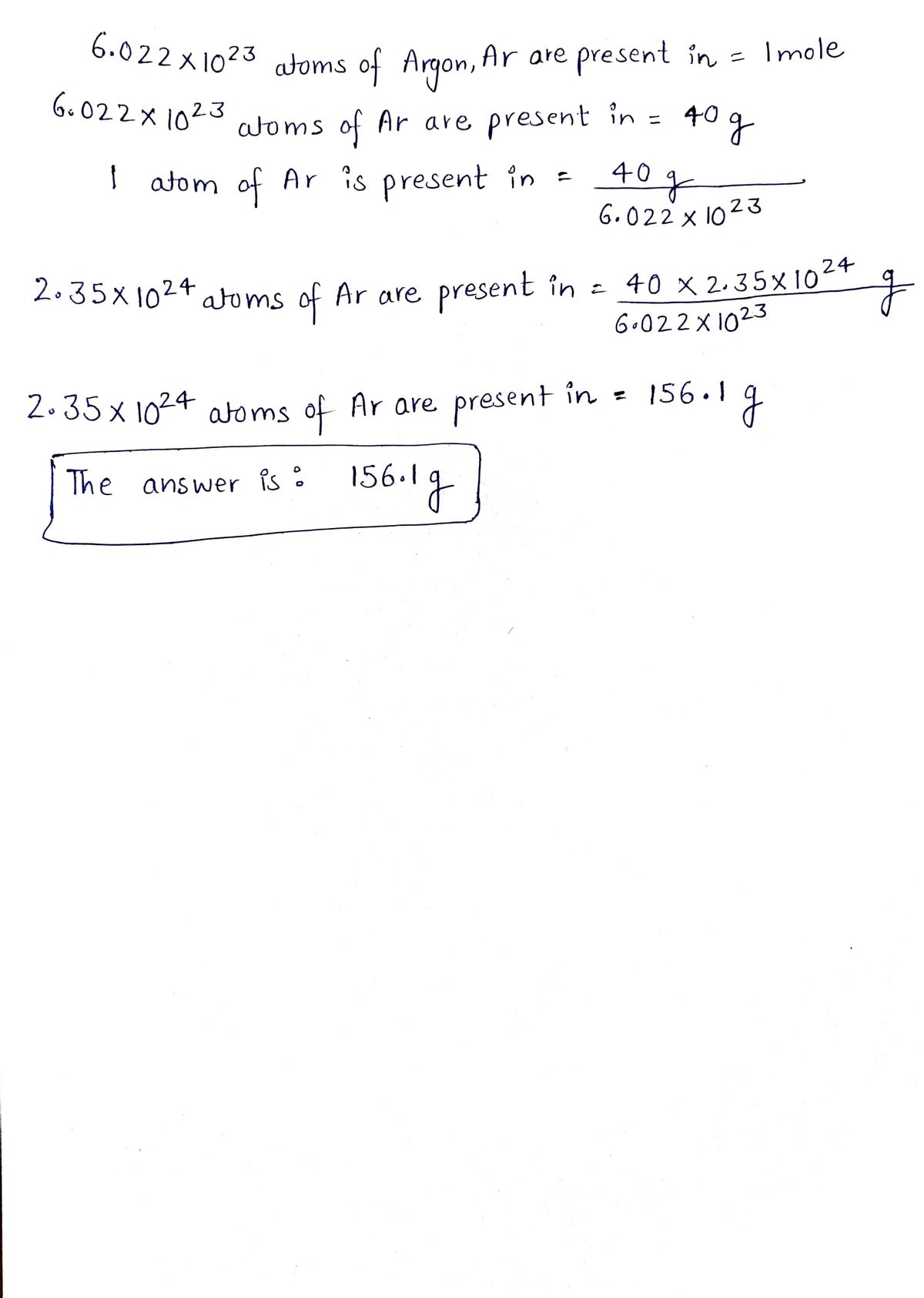
Answered: Set up the following conversion…
Solved The compression factor for a gas is 0.79 at 300 K and
Solved The compression factor (Z) for a real gas can be
Real Gases. The ideal gas equation of state is not sufficient to
If `Z` is a compressibility factor, van der Waals' equation at low
 Under Armour Women's Short HeatGear High Waisted Ankle No-Slip Leggings, (400) Team Royal / / Black, Medium Short : Clothing, Shoes & Jewelry
Under Armour Women's Short HeatGear High Waisted Ankle No-Slip Leggings, (400) Team Royal / / Black, Medium Short : Clothing, Shoes & Jewelry Fitness Leather Weight Lifting Dip Belt with Chain Pull-Ups Squat Back Support
Fitness Leather Weight Lifting Dip Belt with Chain Pull-Ups Squat Back Support xbox series s 1tb
xbox series s 1tb White Plastic Tea Cup, Capacity: 125 Ml, Size: 2.5 Inch at Rs 20/piece in New Delhi
White Plastic Tea Cup, Capacity: 125 Ml, Size: 2.5 Inch at Rs 20/piece in New Delhi How To Draft A Pattern For A Sports Bra
How To Draft A Pattern For A Sports Bra 75 Best Couples Halloween Costumes 2021 - Cute and Funny Couples Costume
75 Best Couples Halloween Costumes 2021 - Cute and Funny Couples Costume