Microbiological Media Management - SOP & Guideline - Pharma Beginners
4.6 (308) In stock

Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.
The importance of growth promotion testing
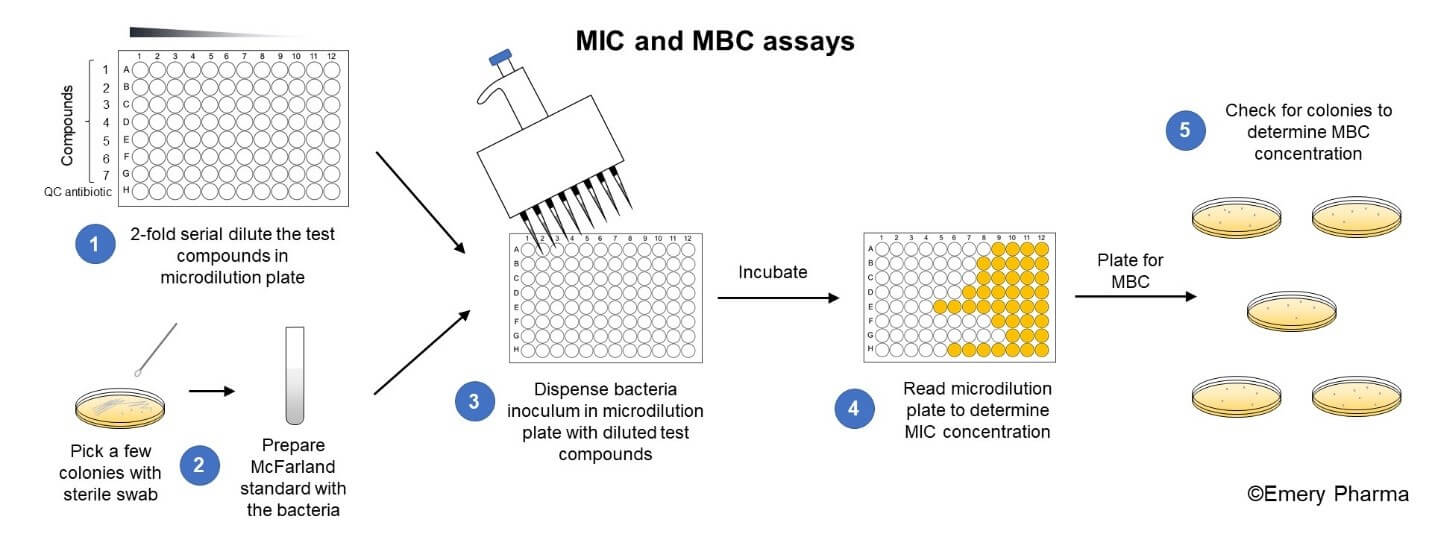
Minimum Inhibitory Concentration (MIC) - Emery Pharma

SOP for Maintenance of Stock Cultures in Microbiology - Pharma Boss

In-process Microbial Control During Aseptic Processing (High-Risk

A Guide On What SOPs are Required for a New Business

SOP of Media Preparation, PDF, Growth Medium
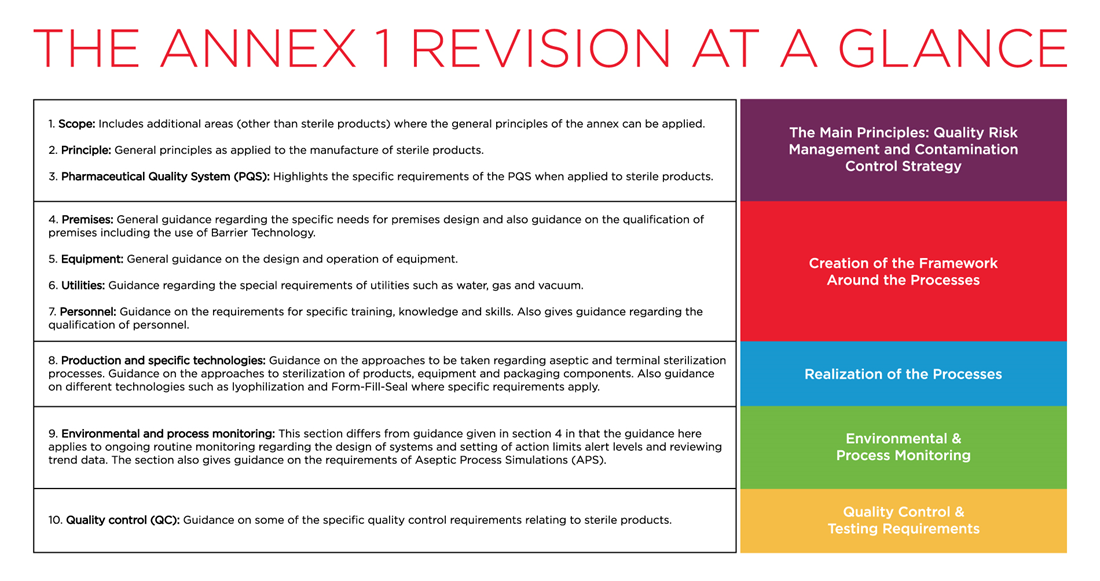
EU GMP Annex 1

Microbial Culture Media Preparation

Validation of Aseptic Processes Using Media Fill

Entry and Exit of Micro Lab Sop, PDF, Clothing

Step wise approach for the Quality Risk Management (QRM) in
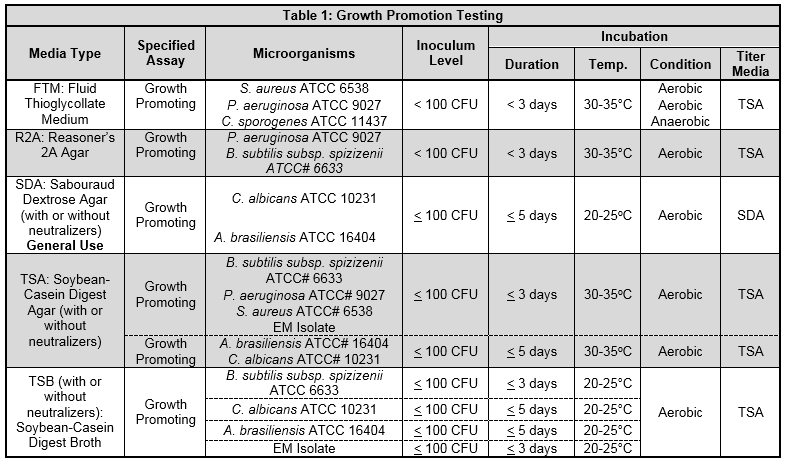
How To Establish Growth Promotion Tests For Pharmaceutical Culture Media

SOP For The Media Prepration For Microbial Analysis
Diagnostics, Free Full-Text

In-process Microbial Control During Aseptic Processing (High-Risk)
PHARMACEUTICAL MICROBIOLOGY: Microbial Growth media Requirements
9.3 Media Used for Bacterial Growth – Microbiology: Canadian Edition
CULTURE (GROWTH) MEDIA - Everything Microbiology
 obsessed with Dawn 🌟 : r/rupaulsdragrace
obsessed with Dawn 🌟 : r/rupaulsdragrace- Nike Sportswear Club Fleece Embroidered Hoodie
- Perfume Feminino Calvin Klein Edp 2023 Eternidade Para Mulheres Verão - Calvin Klein
 Cinnamon Oat & Nut Clusters - The Healthy Home Cook
Cinnamon Oat & Nut Clusters - The Healthy Home Cook Women's Ripstop Longline Puffer Jacket in Khaki Grid
Women's Ripstop Longline Puffer Jacket in Khaki Grid Beginner Yoga Pack Yoga for kids, Yoga for beginners, Kids yoga
Beginner Yoga Pack Yoga for kids, Yoga for beginners, Kids yoga
)
