FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice
4.6 (718) In stock

The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

Medtronic Diabetes receives FDA warning over quality system

Drug Delivery Business News on LinkedIn: Baxter recalls some syringe pumps due to underdosing risk

FDA Issues Class I Recall of Certain Medtronic Insulin Pumps

Medtronic MiniMed Insulin Pump Lawsuit March 2024 - Select Justice

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump
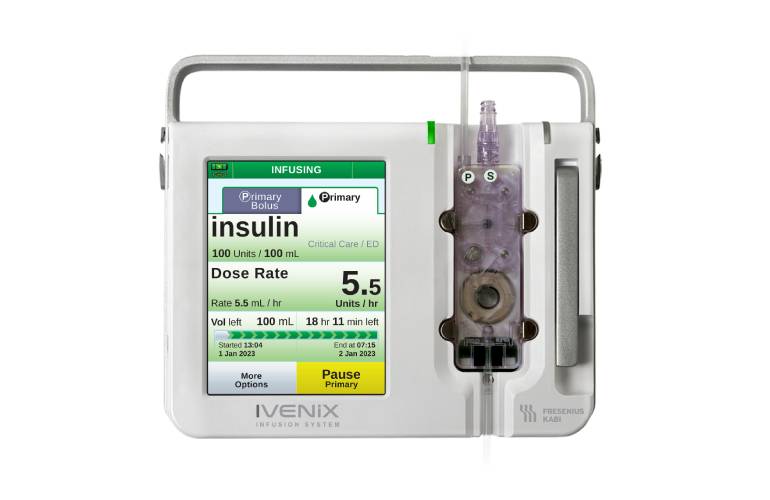
Fresenius Kabi has a Class I Ivenix infusion pump recall

What's Next for Tandem? T:slim & Beyond – Diabetes Connections

Medtronic implanted drug pump gets a second class I recall

Certain Medtronic Mini-Med 600 Series Insulin Pumps Recalled

Baxter warns on some infusion pumps due to potential false alarms

Insulin pump recall: MiniMed pumps recalled by FDA

Medtronic MiniMed Insulin Pump Lawsuit

Medtronic MiniMed Insulin Pump Lawsuit
Insulin Pump: What It Is, How It Works & Types
Omnipod Insulin Pump Therapy, Simplified
Insulin Pump Program - Northeast Georgia Health System
 Xmas Dogs Children's Cotton Jersey Leggings – Rainbows & Sprinkles
Xmas Dogs Children's Cotton Jersey Leggings – Rainbows & Sprinkles Skims Soft Lounge Tank Top
Skims Soft Lounge Tank Top 78 Up All Night With Rhonda Shear Stock Photos, High-Res Pictures, and Images - Getty Images
78 Up All Night With Rhonda Shear Stock Photos, High-Res Pictures, and Images - Getty Images PIKADINGNIS Clearance Womens Thermal Underwear Autumn Winter Long Johns High Collar Keep Warm Slim Ladies Sexy Thermal Underwear Girls
PIKADINGNIS Clearance Womens Thermal Underwear Autumn Winter Long Johns High Collar Keep Warm Slim Ladies Sexy Thermal Underwear Girls Freya Women's Active Performance Capri Pant, Black, X-Small : : Clothing, Shoes & Accessories
Freya Women's Active Performance Capri Pant, Black, X-Small : : Clothing, Shoes & Accessories Bras Plus Size Push Up Women Deep Cup Bra Concentrated Hide Back Fat Underwear Shaper Incorporated Full Coverage Lingerie From Tchai, $21.14
Bras Plus Size Push Up Women Deep Cup Bra Concentrated Hide Back Fat Underwear Shaper Incorporated Full Coverage Lingerie From Tchai, $21.14