Real gases
4.7 (355) In stock


SOLUTION: Gases 9a real gases non ideal - Studypool
gas laws - Volume occupied by real gases - Chemistry Stack Exchange

Deviations from Ideal Gas Law Behavior

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

Important Differences Between Ideal Gas and Real Gas – intactone

Why do real gases not behave exactly like ideal gases? Real gases are always hotter than ideal gases.
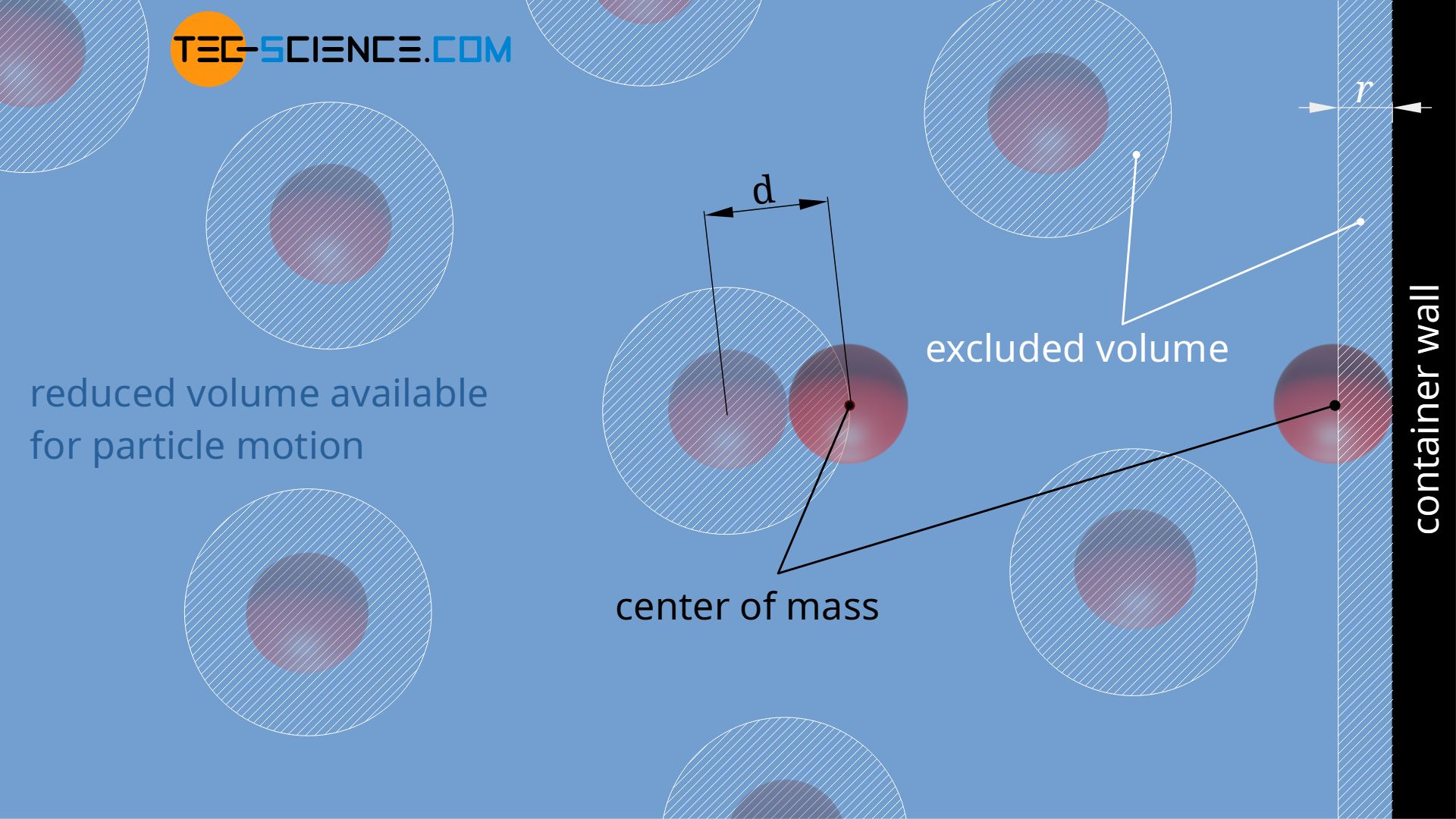
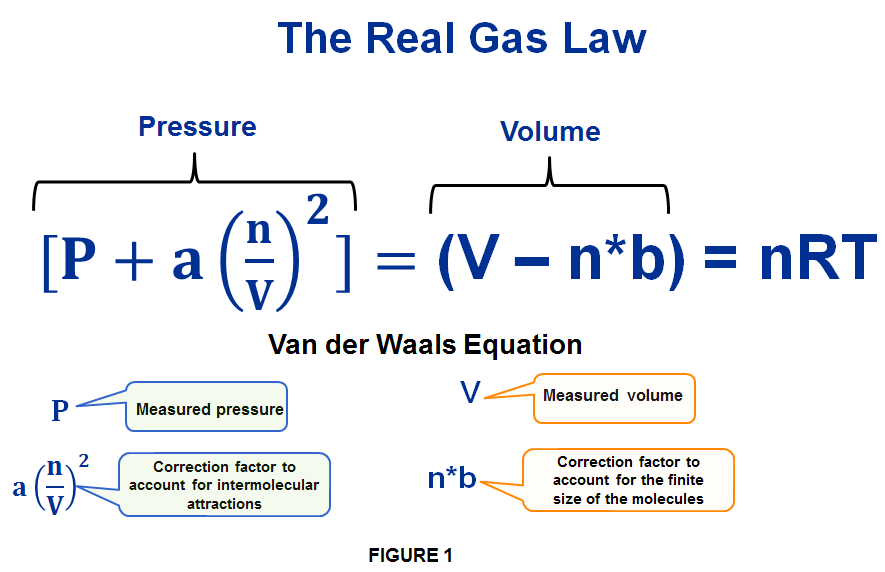
Van der Waals equation (gas law for real gases) - tec-science
How does temperature affect real gas? - Quora

Real Gases vs Ideal Gases & the Compressibility Factor

4.5 Real Gases, Gas Laws: Pressure, Volume, and Temperature

The behavior of real gases in terms of reduced pressure.
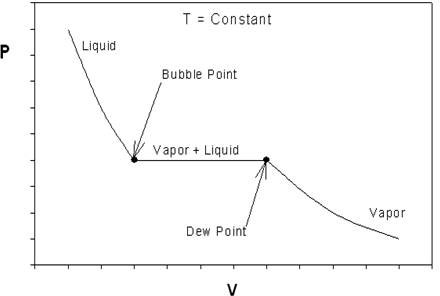
Real Gases PNG 520: Phase Behavior of Natural Gas and Condensate Fluids

Chemistry 231 Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart. - ppt download
THE 3rd STATE OF MATTER – What is a Real Gas? – Computer Aided Design & The 118 Elements

For real gases van der Waals equation is written as pane (v - nb) = n RT, where 'a' and 'b' are van der Waals constants. Two sets of gases are: (1)
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
COMPRESSION AND EXPANSION OF GASES – Chemical Engineering Projects
Compression Factor Exam Problem using Molar Volumes - Fully Explained!
The compression factor (compressibility factor) for 1 mol of a van der
The Compression Factor, Z, and Real Gases - What you NEED to Know





