Sacituzumab Earns Regular FDA Approval for TNBC - NCI
4.5 (740) In stock

Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.
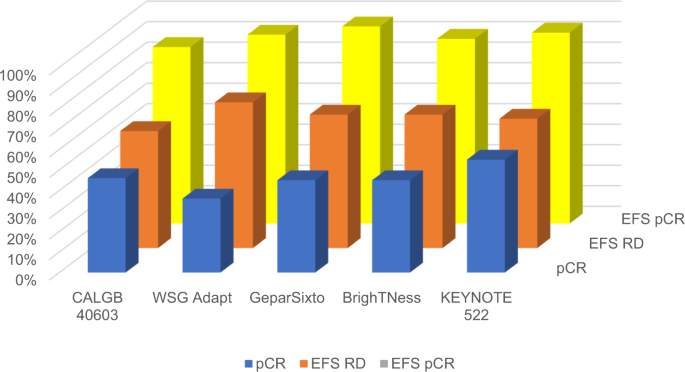
Triple negative breast cancer: Pitfalls and progress

Targeted therapy for breast cancer: An overview of drug classes
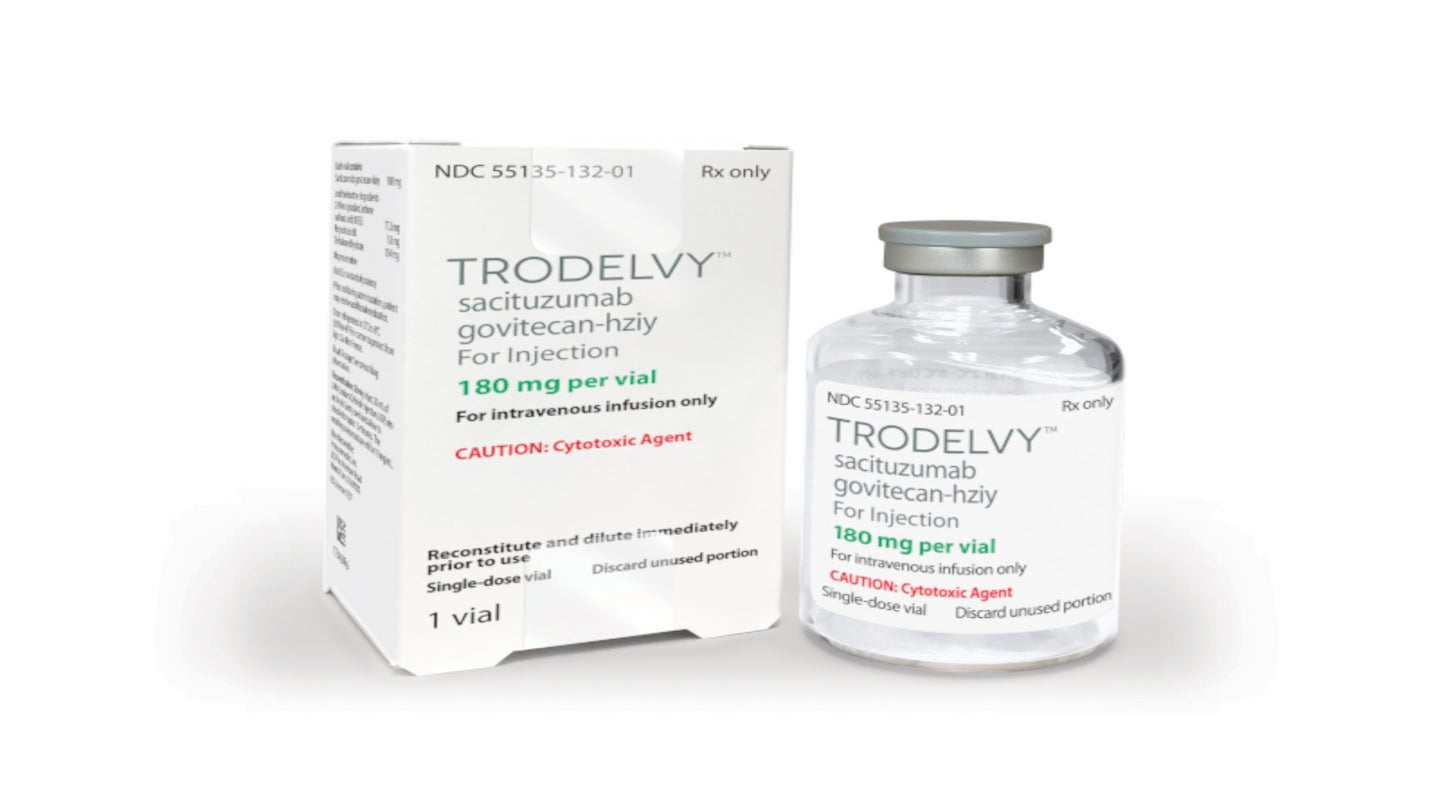
Trodelvy for the Treatment of Advanced Triple-Negative Breast Cancer

A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer - ScienceDirect
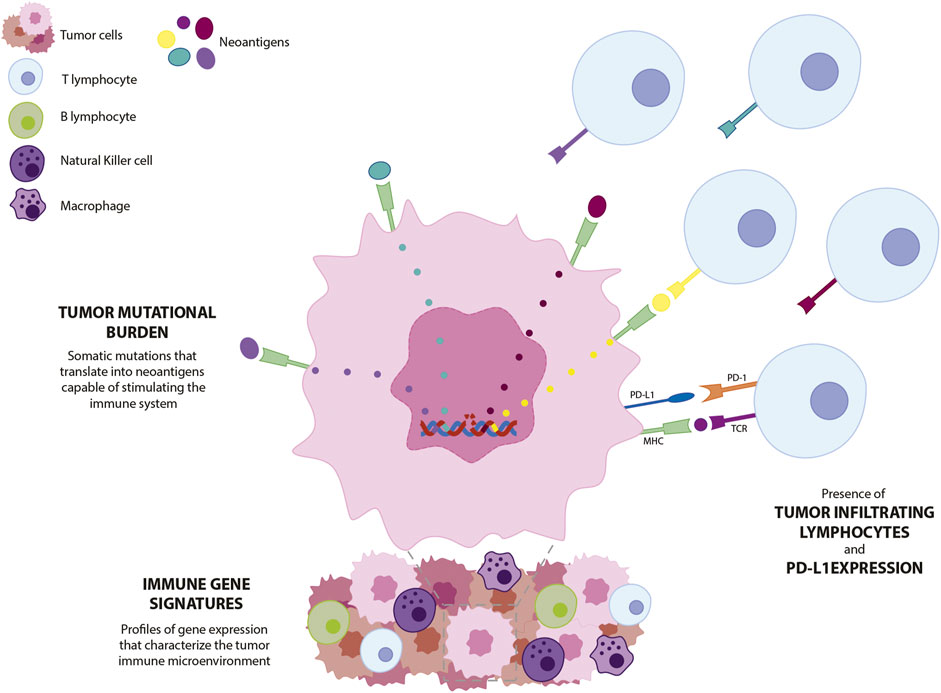
Frontiers Immunotherapy in triple-negative breast cancer

Sacituzumab Earns Regular FDA Approval For TNBC NCI
Clinical Review - Sacituzumab Govitecan (Trodelvy) - NCBI Bookshelf

Alexis Johnson on LinkedIn: So grateful to be presenting one of my
View of Sacituzumab Govitecan (Trodelvy) Canadian Journal of Health Technologies
Sacituzumab Earns Regular FDA Approval for TNBC - NCI
380 vs. 38 Special - What's the Difference & Which is Better?
Blogue Expressão: 25 de abril 40 anos - 38 - A moda em 1974
Novidade em SST: NR 38 para limpeza urbana e manuseio de resíduos sólidos
Google Pixel 8 Pro Display test - DXOMARK
De 38 a arminha d´água: a polêmica história do emoji de pistola - Canaltech
 PANTALÓN DE TRABAJO MULTIBOLSILLOS PARA MUJER - Unifardas
PANTALÓN DE TRABAJO MULTIBOLSILLOS PARA MUJER - Unifardas No Wire / Comfort Bras – GoodNight GoodMorning
No Wire / Comfort Bras – GoodNight GoodMorning Guitarra Strato Street ST-111 Branca Waldman
Guitarra Strato Street ST-111 Branca Waldman Yuemengxuan Women Cycling Suit, Girls Splicing Long Sleeve Zipper Coat + Tight Pants
Yuemengxuan Women Cycling Suit, Girls Splicing Long Sleeve Zipper Coat + Tight Pants Gorilla Wear Augustine Old School Workout Top
Gorilla Wear Augustine Old School Workout Top Love Apple Farm's Tomato Variety Photo Album: All colors of
Love Apple Farm's Tomato Variety Photo Album: All colors of