Show that the van der Waals equation leads to values of Z <
4.8 (427) In stock

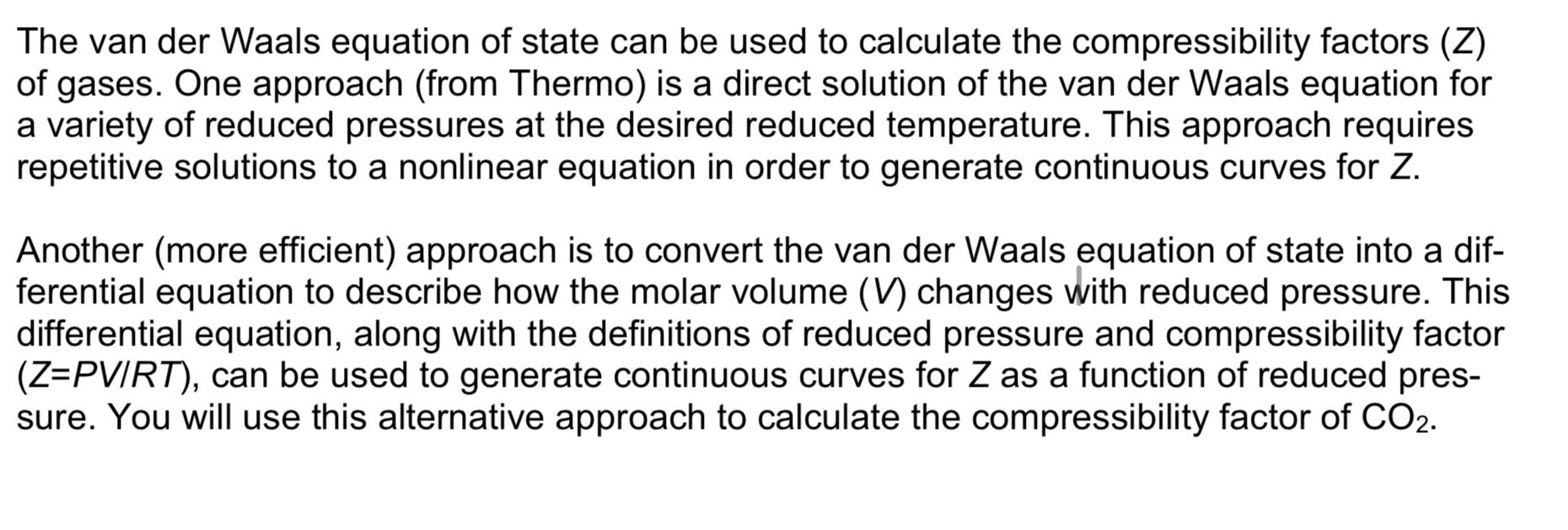
Solved The van der Waals equation of state can be used to
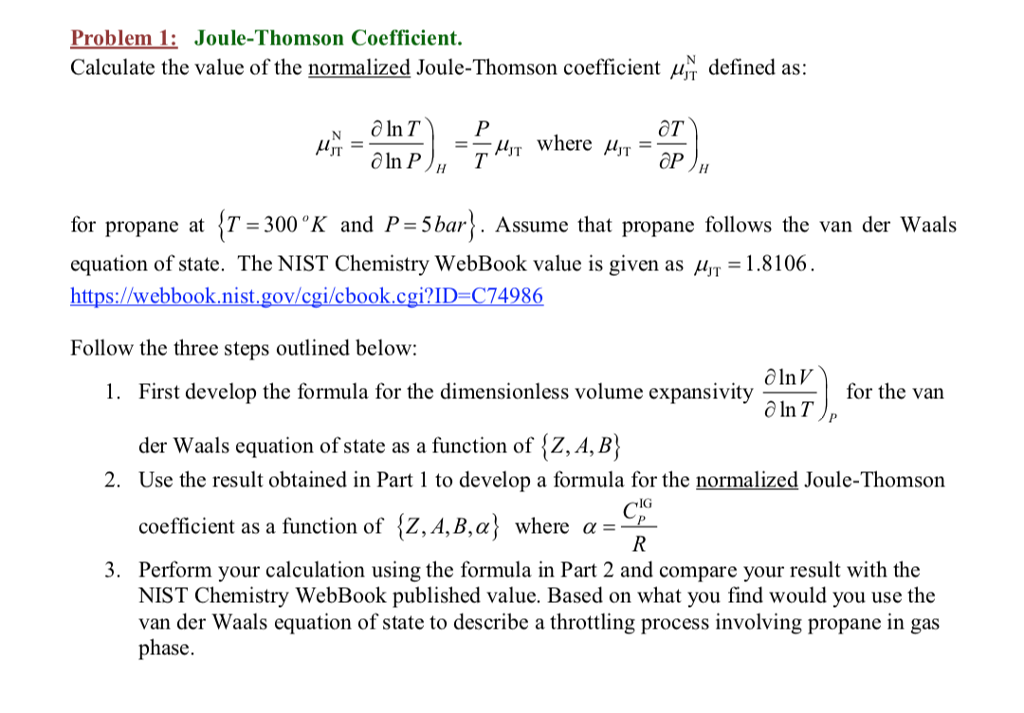
Solved Problem 1: Joule-Thomson Coefficient Calculate the

38 1 THE PROPERTIES OF GASES Discussion PDF, PDF, Gases

SOLVED: Use the van der Waals Eq. in terms of reduced quantities to derive the condition for Z > 1, and Z < 1, respectively. Use an expression of TR in terms of VR.

Use the van der Waals equation and the ideal gas equation to calc
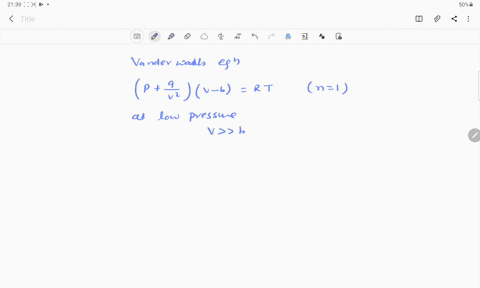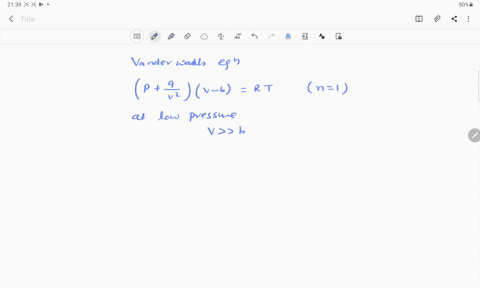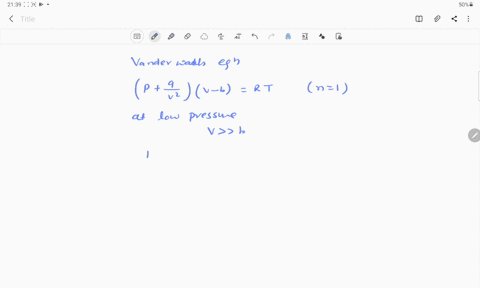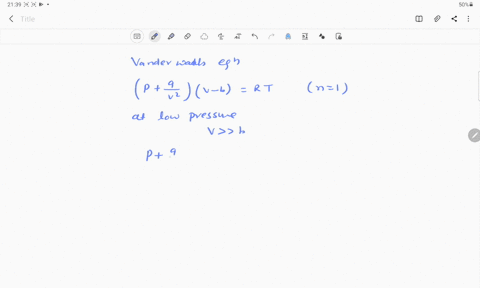
⏩SOLVED:If Z is a compressibility factor, van der Waals equation at…
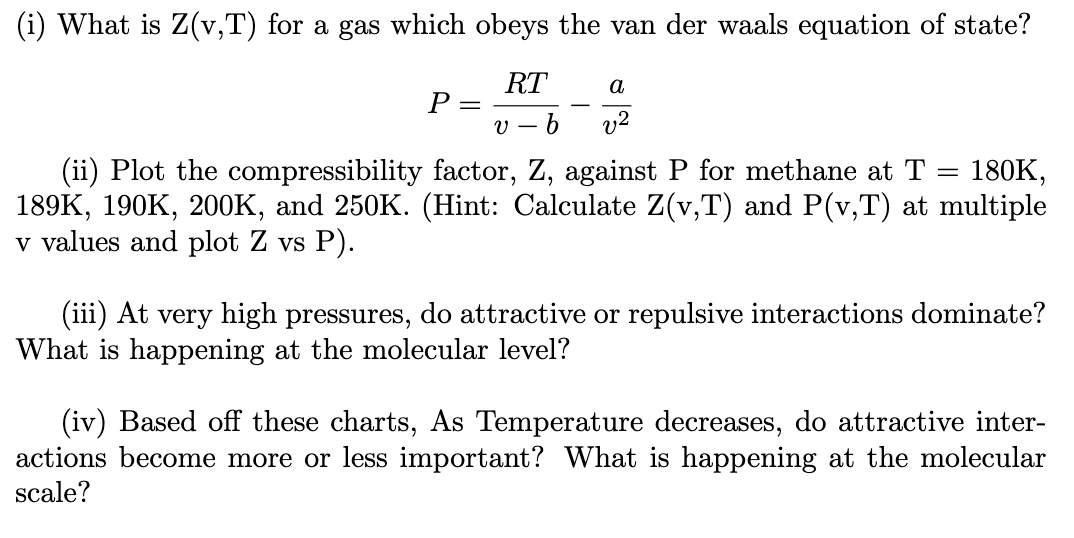
Solved (i) What is Z(v,T) for a gas which obeys the van der

Non-Ideal Gas Behavior Chemistry: Atoms First

Use the van der Waals equation and the ideal gas equation to calc

PDF) 38 1 THE PROPERTIES OF GASES Discussion questions
Answered: Compression factor of a gas with van…
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
Solved Z = 4. We saw in class that the compression factor
SOLVED: Derive an expression for the compression factor of a gas
 Realistic Grey Orange Cat Figurine Set Perfect Educational - Temu
Realistic Grey Orange Cat Figurine Set Perfect Educational - Temu- Wonderbra Ultimate backless plunge push up bra in beige
 Mastectomy Bra Set Pocket Bra with Lace Embroidery Wire Free for
Mastectomy Bra Set Pocket Bra with Lace Embroidery Wire Free for Raio X: Quanto custa manter um Chevrolet Onix LTZ 2024 de R
Raio X: Quanto custa manter um Chevrolet Onix LTZ 2024 de R Wholesale Adjustable Pants Seamless Fitness in Stock Gym Push up
Wholesale Adjustable Pants Seamless Fitness in Stock Gym Push up No Boundaries Juniors Plus Size Flare Pants, 2-Pack, Sizes 1X-4X
No Boundaries Juniors Plus Size Flare Pants, 2-Pack, Sizes 1X-4X
