Solved 2. (20 points) At low pressures, the compressibility
4.6 (275) In stock


Solved Problem 2. ( 30 points) The ideal gas law can
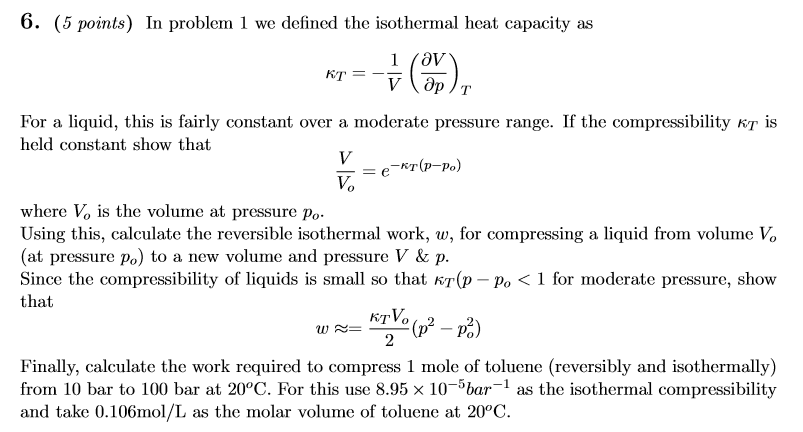
Solved In problem 1 we defined the isothermal heat capacity

Gas Compressibility Factor and Control Valve Sizing

Isothermal compressibility of the solution, β T × 10 5 , bar -1

Thermodynamics: An Engineering Approach - 5th Edition - Part II by

Solved QUESTION 2. Using the equation below, calculate the
Ethylene Glycol Heat-Transfer Fluid Properties

In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
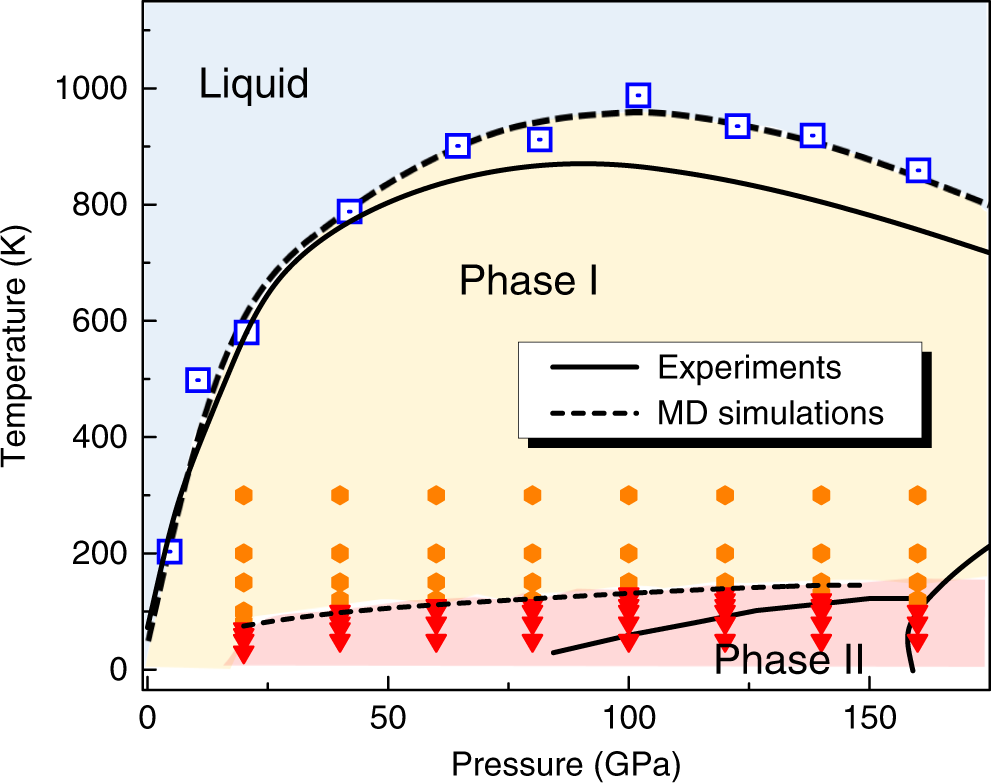
Understanding high pressure molecular hydrogen with a hierarchical machine-learned potential
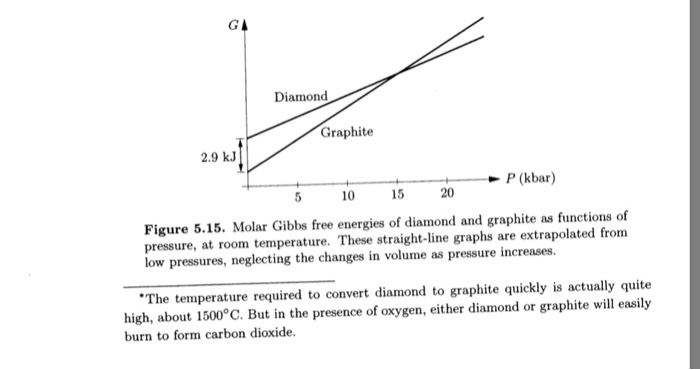
Solved 2. (20 points) You can describe how compressible a
Isothermal compressibility κ as a function of temperature at same
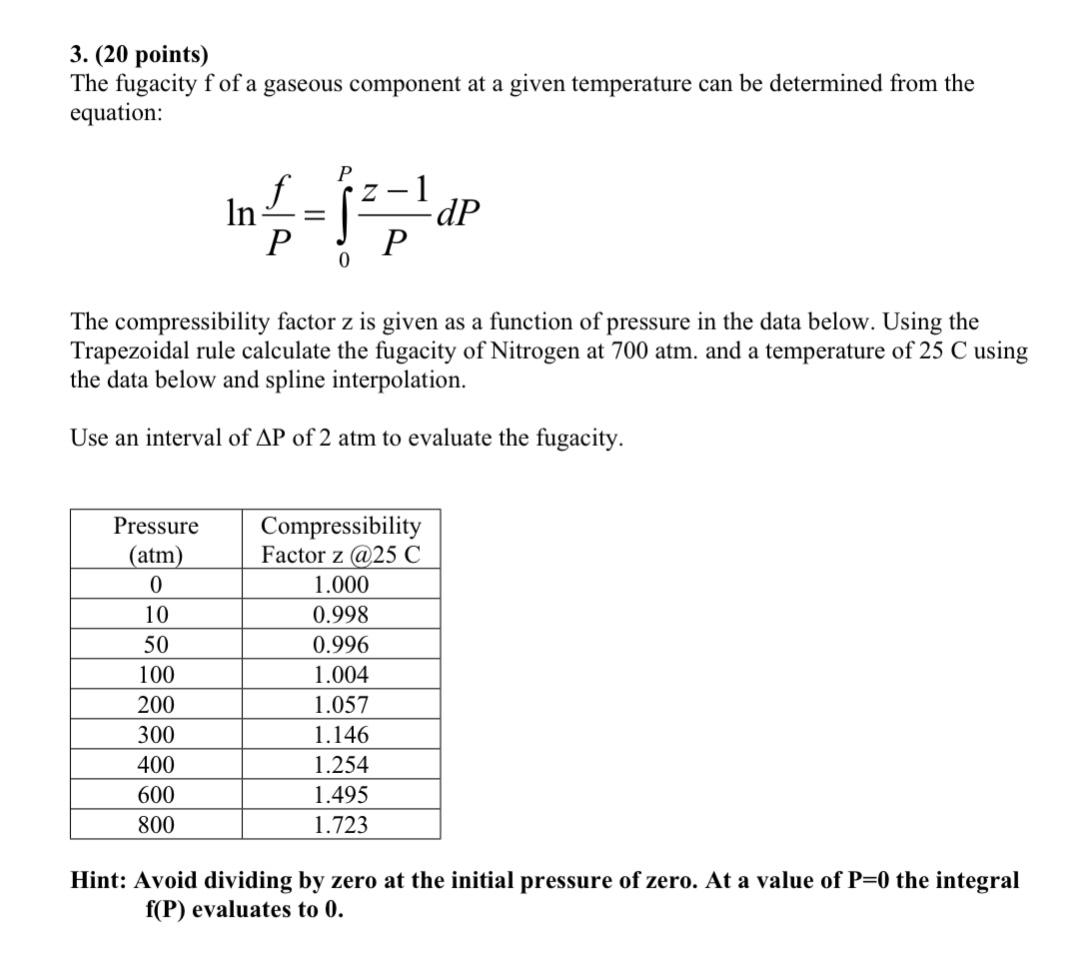
Solved 3. (20 points) The fugacity f of a gaseous component
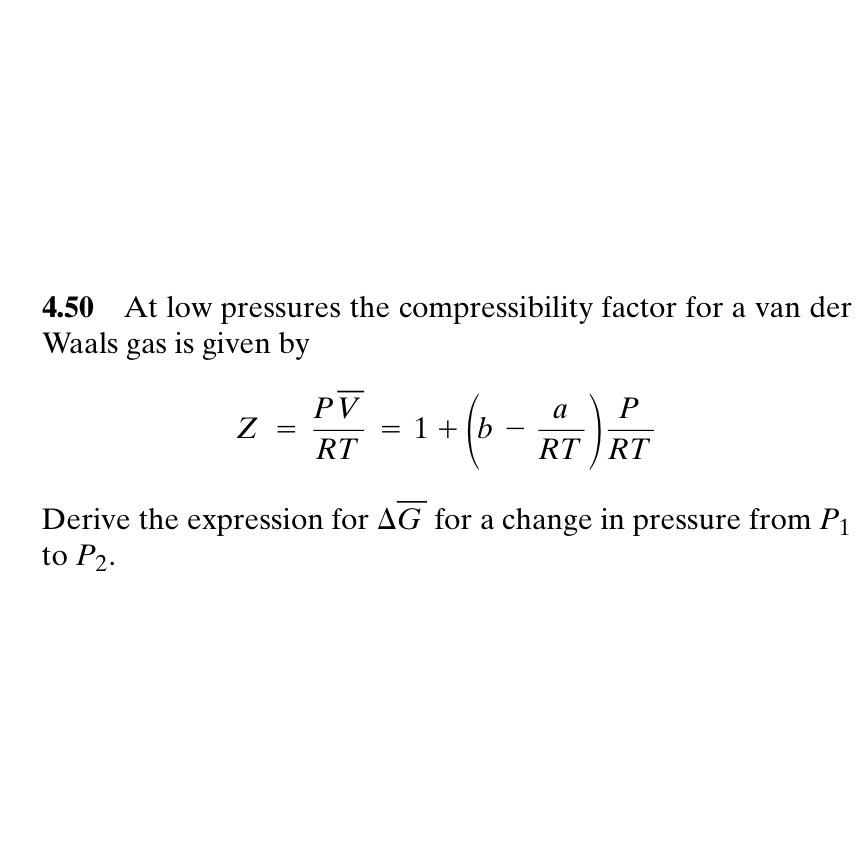
Solved 4.50 At low pressures the compressibility factor for
The value of compression factor at the critical state of a vander waals gas is
Explain how the compression factor varies with pressure and
The compressibility factor a real gas high pressure is:-1 - frac
 Cotopaxi Roso Tights - Womens, FREE SHIPPING in Canada
Cotopaxi Roso Tights - Womens, FREE SHIPPING in Canada Micro HD & Mini HDMI-compatible male to HDMI--compatible Thin
Micro HD & Mini HDMI-compatible male to HDMI--compatible Thin Swimsuits For All Women's Plus Size Loop Strap Blouson Tankini Set With Cargo Short 10 Engineered Floral, Black
Swimsuits For All Women's Plus Size Loop Strap Blouson Tankini Set With Cargo Short 10 Engineered Floral, Black- No filters just pure neat braids done with a lot of love ❤️❤️
 Mueller Adjustable Back & Abdominal Support
Mueller Adjustable Back & Abdominal Support- Matchball Displays on X: 20/22 Brazil 2014 Adidas Brazuca / X

