Solved An ideal gas initially at Pi, V;, and T; is taken
5 (155) In stock

An ideal gas is initially P_1, V_1 is expanded to P_2, V_2 and then compressed adiabatically to the same volume V_1 and pressure P_3. If W is the net work done by

Thermodynamics problems

Isobaric Process - an overview
Solved An ideal gas initially at Pi, Vi, and Ti is taken

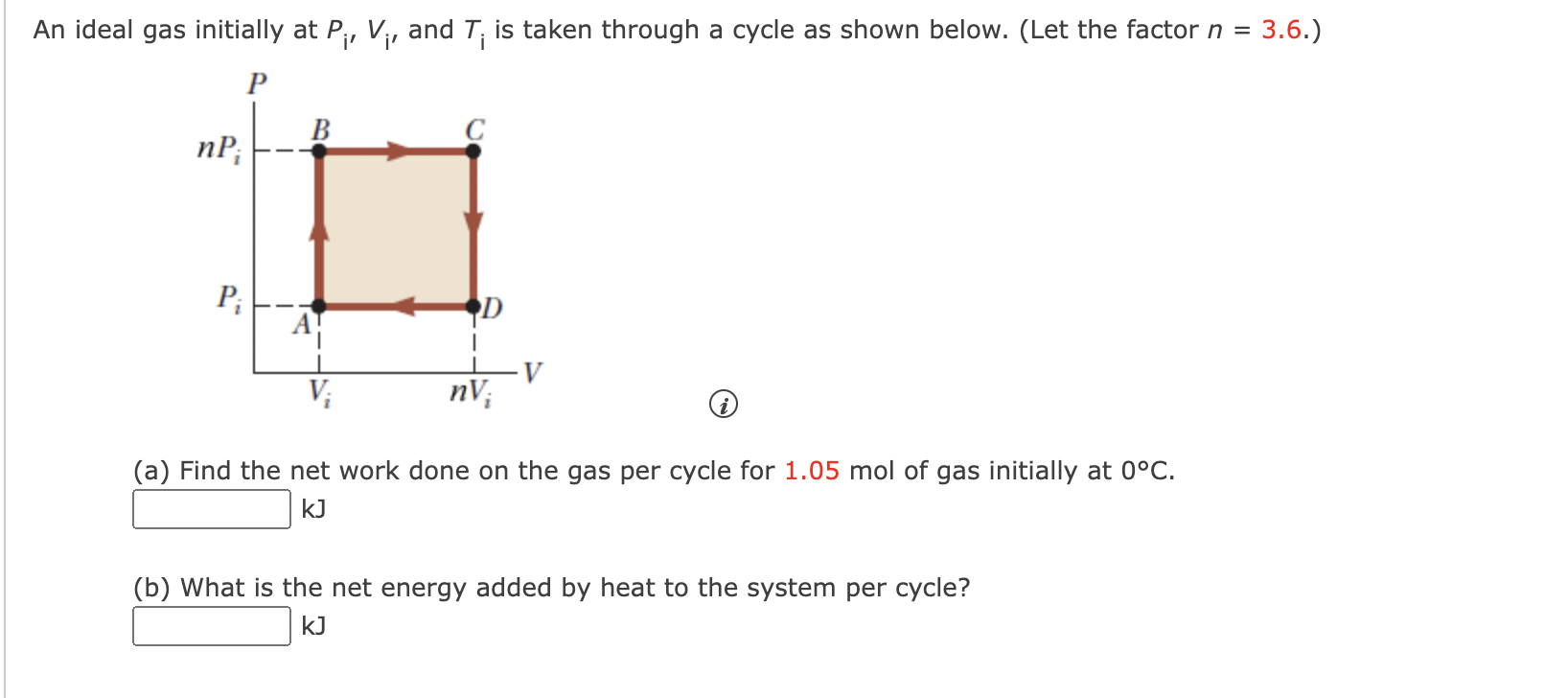
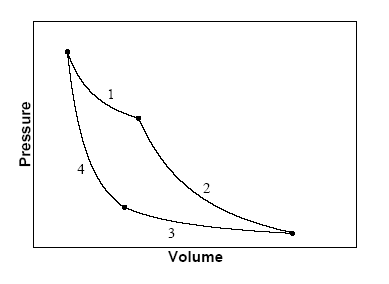
An ideal gas initially at P_i, V_i and T_i is taken through a cycle as shown below. Let the factor n = 3.7. a. Find the net work done on the gas

An ideal gas is taken through the cycle `AtoBtoCtoA,` as shown in the figure, If the net heat
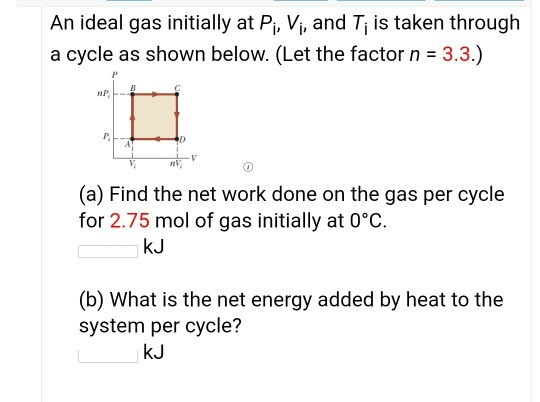
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n = 3.8.) (a) Find the net work done on the

solution manual for applied petroleum reservoir engineering by craft by kholoud hamad - Issuu
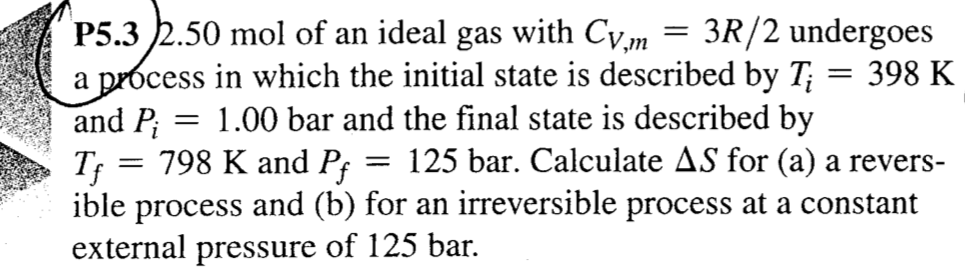
Solved P5.32.50 mol of an ideal gas with Cv 3R/2 undergoes a
entropy
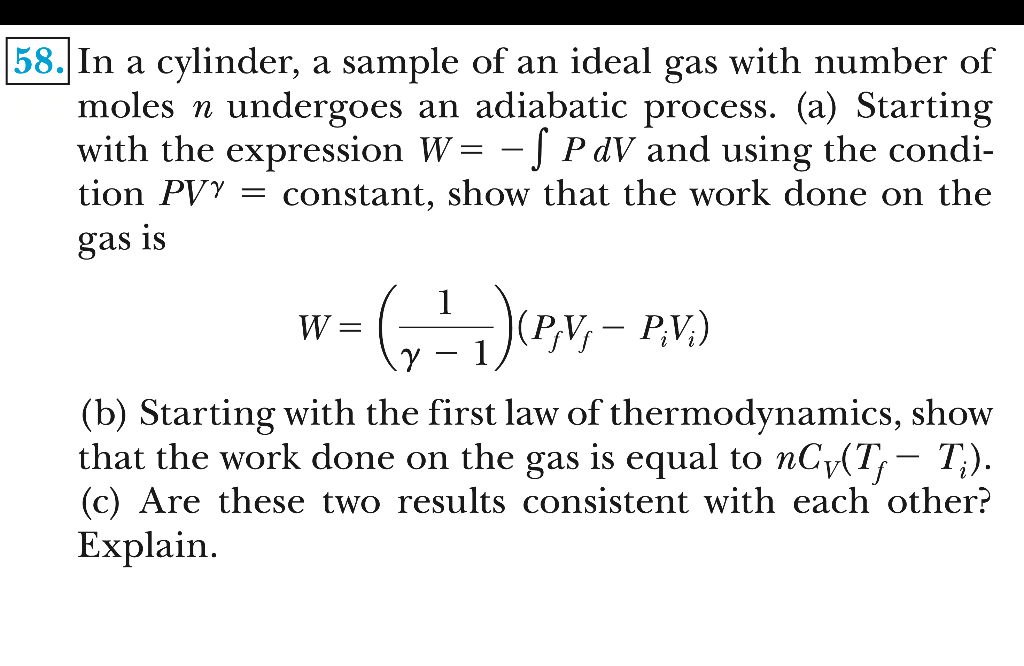
Solved In a cylinder, a sample of an ideal gas with number

One mole of a monoatomic ideal gas initially at a pressure of 2.00 bar and a temperature of 273 K is

A 1.0 mol of ideal gas, initially at 10 atm and 300 K isallowed to expand isothermally to 1.0 atm, as
Ideal Deepness II by PI Studio | Liquid Acrylic Art
70-year-old patient. PI = 41°, so ideal Roussouly-type is 1. Ideal
Givenchy Pi 3.3 oz EDT Spray mens cologne 100 ml NIB 3274878222568
 Travel and Packing Checklists PLR : Simply Couture PLR
Travel and Packing Checklists PLR : Simply Couture PLR- Womens Fitness Clubs of Canada (@womensfitnessofficial) • Instagram photos and videos
 Playtex Women's Sz 36D 18 Hour Ultimate Shoulder Comfort Wireless
Playtex Women's Sz 36D 18 Hour Ultimate Shoulder Comfort Wireless KBODIU Everyday Bras for Women, Plus Size Comfort Bras, Women's Ultimate Lift Wirefree Bra Comfortable Lace Breathable Bra Underwear No Rims Bras No Underwire Wine
KBODIU Everyday Bras for Women, Plus Size Comfort Bras, Women's Ultimate Lift Wirefree Bra Comfortable Lace Breathable Bra Underwear No Rims Bras No Underwire Wine La Gaunche, Black, Bamboo – Blue Sky Clothing Co Ltd
La Gaunche, Black, Bamboo – Blue Sky Clothing Co Ltd__74421.1669178841.jpg?c=2) Alpine Swiss Vance Mens Wool Blend Button Up Coat - Alpine Swiss
Alpine Swiss Vance Mens Wool Blend Button Up Coat - Alpine Swiss
