Solved Show that the compressibility factor of van der Waals
5 (571) In stock

Answer to Solved Show that the compressibility factor of van der Waals

Van Der Waals Equation of State - an overview
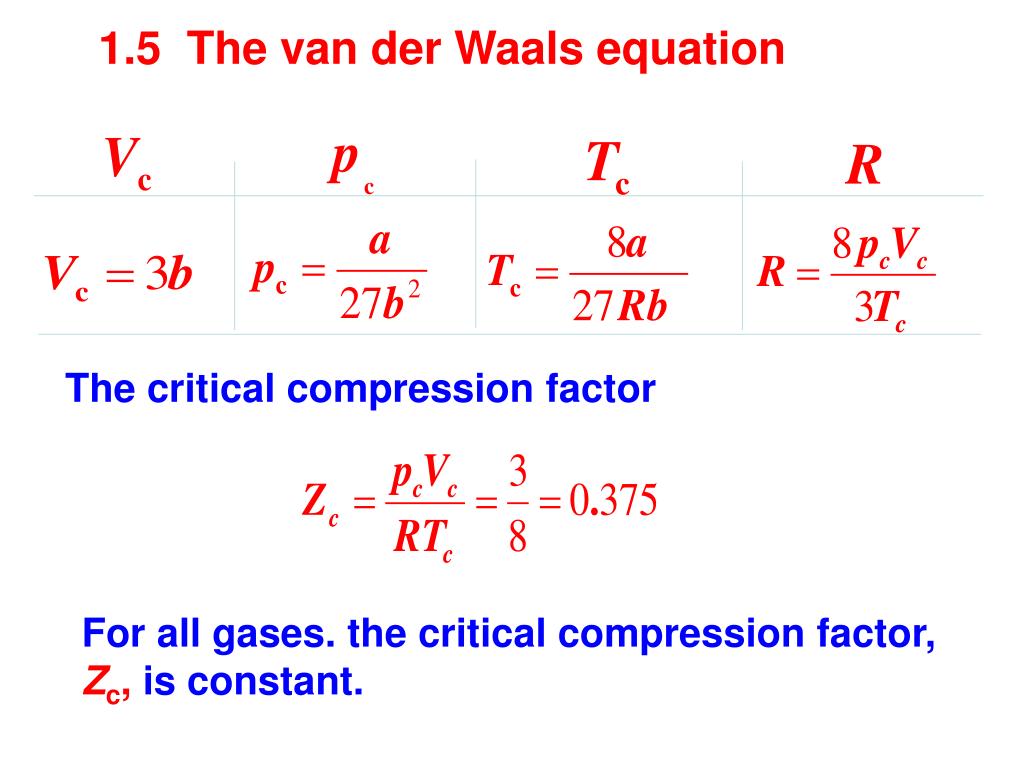
PPT - Real gases PowerPoint Presentation, free download - ID:3959491

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

PDF) A Modified Form of the van der Waals Equation of State
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

6.3: Van der Waals and Other Gases - Physics LibreTexts

physical chemistry - Compressibility Factor Graph - Which gas attains a deeper minimum? - Chemistry Stack Exchange

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

Solved Show that the compressibility factor of van der Waals

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0-b) = RT may be written as (P+*}() =RT of PV + 9 =RT of PV=RT - For large V (at very

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

Solved The van der Waals equation of state can be used to

Deviations from Ideal Gas Law Behavior
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
Solved Real gas effects can be expressed as departures from
Figure 3 from A Simple Equation Of State For Calculating The
 ZHAGHMIN Tie Dye Leggings for Women Leggings Glitter Bling Shiny Leg Sequin Pants Casual Yoga Leggings Slim for Holiday Women'S Sequin Outfits Yoga Pants Womens Yoga Pants Petite With Pockets Dress
ZHAGHMIN Tie Dye Leggings for Women Leggings Glitter Bling Shiny Leg Sequin Pants Casual Yoga Leggings Slim for Holiday Women'S Sequin Outfits Yoga Pants Womens Yoga Pants Petite With Pockets Dress So Obsessed Smooth Push-Up Bra
So Obsessed Smooth Push-Up Bra- Parachute Pants curated on LTK
 Kids Girls #Selfie Tracksuit Baby Pink Hooded Hoodie Bottom Jogging Suit Joggers Age 5 6 7 8 9 10 11 12 13 Years
Kids Girls #Selfie Tracksuit Baby Pink Hooded Hoodie Bottom Jogging Suit Joggers Age 5 6 7 8 9 10 11 12 13 Years Qué material necesito para el entrenamiento funcional - ISAF
Qué material necesito para el entrenamiento funcional - ISAF Hand Block Yellow Pink Printed Kurti With Afghani Pants
Hand Block Yellow Pink Printed Kurti With Afghani Pants