Solved The virial expansion of the compression factor (Z)
4.6 (624) In stock

Virial expansion - Wikipedia
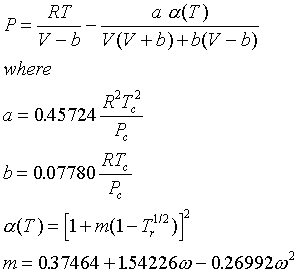
Thermodynamic Models

CH 353 Lecture 4: Virial Expansion and Cubic VdW - OneClass
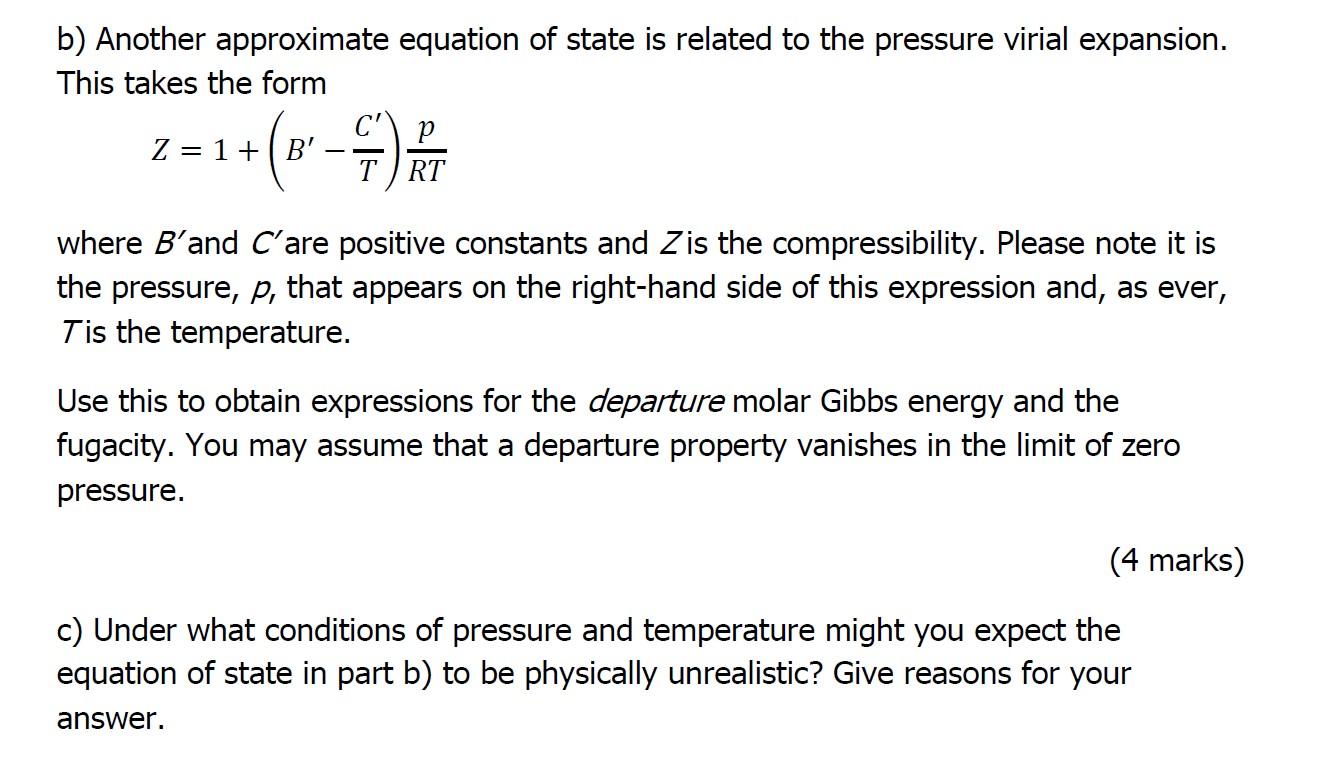
Solved b) Another approximate equation of state is related

Virial Coefficient - an overview

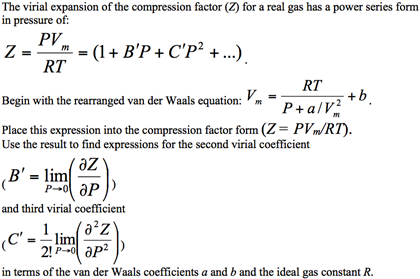
The virial form of van der Waal's gas equation is PV=RTleft(1+

Degrees Conferred by Major ( ) Source: National Center for

Virial Equation of State2, PDF, Physical Chemistry
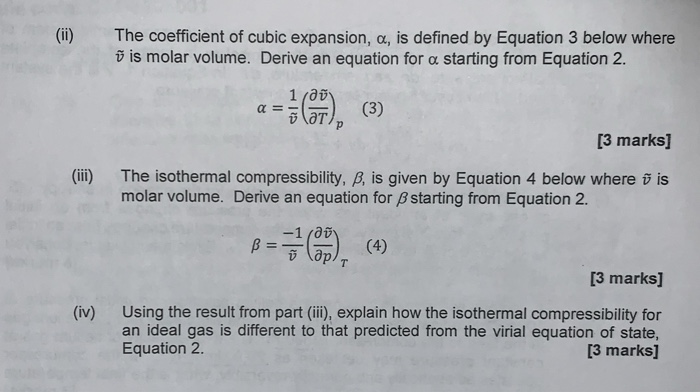
Solved (b) The virial equation of state for a gas, including

20170214160241lecture 2 Skm3013-Virial and Graphical

The Compression Factor, Z, and Real Gases - What you NEED to Know
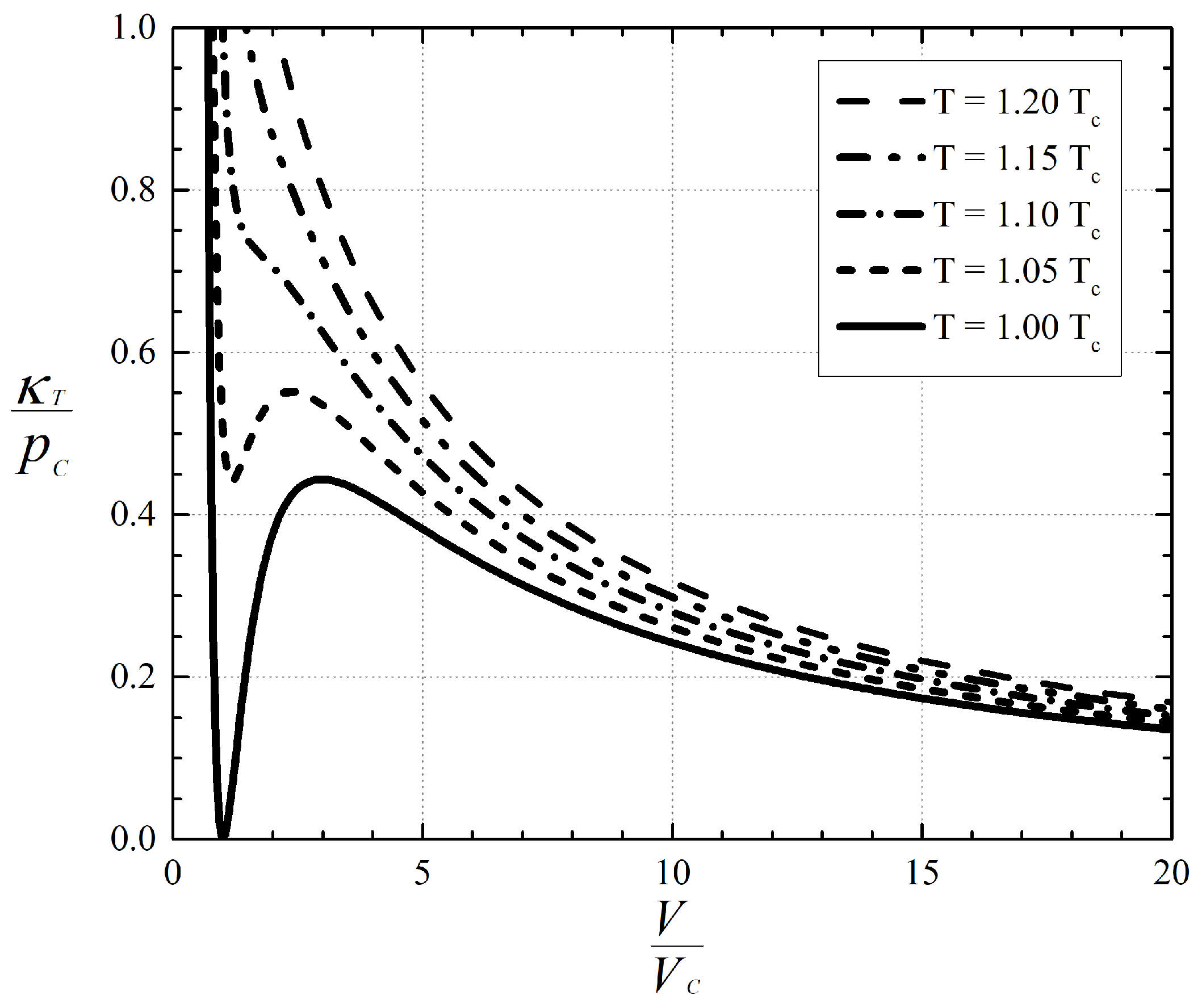
Fluids, Free Full-Text

Solved The compression factor is given by Z = pV/RT = 1 +
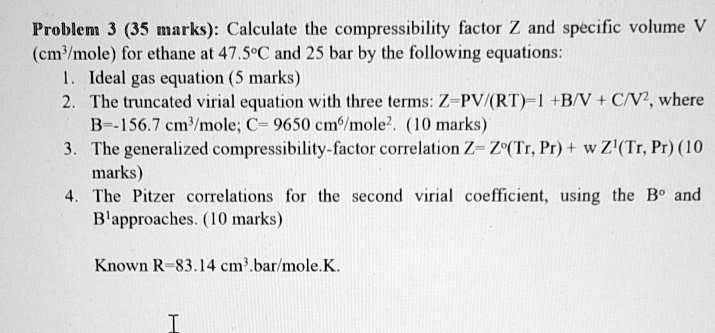
SOLVED: Problem 3 (35 marks): Calculate the compressibility factor Z and specific volume V cm/mole for ethane at 47.5°C and 25 bar by the following equations: 1. Ideal gas equation - 5
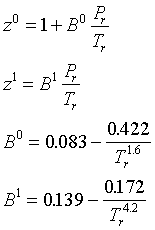
Thermodynamic Models
Solved 9 Compression factor Z Use the van-der-Waals equation
COMPRESSION AND EXPANSION OF GASES – Chemical Engineering Projects
Show that the van der Waals equation leads to values of Z <
If `Z` is a compressibility factor, van der Waals' equation at low
 Tawop Front Buckle Sexy Gathe R Up Breast Milk Sleep Lace No Steel Ring Bra 36Ddd Bras For Women Lily
Tawop Front Buckle Sexy Gathe R Up Breast Milk Sleep Lace No Steel Ring Bra 36Ddd Bras For Women Lily Sleep Is the Cousin by Vega7 the Ronin & Superior (Album, Hardcore Hip Hop): Reviews, Ratings, Credits, Song list - Rate Your Music
Sleep Is the Cousin by Vega7 the Ronin & Superior (Album, Hardcore Hip Hop): Reviews, Ratings, Credits, Song list - Rate Your Music Shiny Yoga Pants for Women Tall Tie Dyed High Waisted Seamless Tie Dye Yoga Fitness Girls Yoga Pants Size 8 : Ropa, Zapatos y Joyería
Shiny Yoga Pants for Women Tall Tie Dyed High Waisted Seamless Tie Dye Yoga Fitness Girls Yoga Pants Size 8 : Ropa, Zapatos y Joyería AYBL Balance V2 Seamless Shorts Chambray Blue
AYBL Balance V2 Seamless Shorts Chambray Blue Seven7 Women's Fashion Jean (Affirmation, 16)
Seven7 Women's Fashion Jean (Affirmation, 16) Ileostomy bag - 411631 - Unomedical - children
Ileostomy bag - 411631 - Unomedical - children