Five Common Mistakes Submitting a Premarket Notification
4.7 (310) In stock

How you can avoid the most common errors made when submitting a 510(k), the “premarket notification,” with simple measures

Navigating the Regulatory Submission Process

Premarket Notification The 510(k) Process
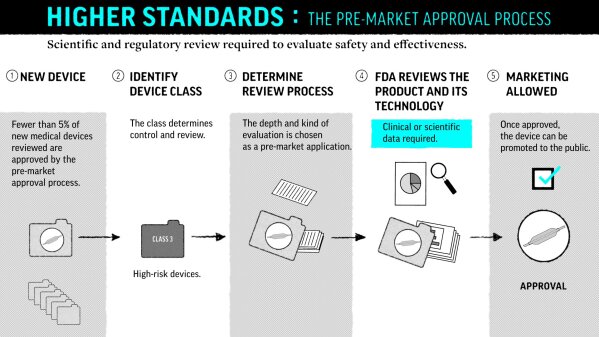
At FDA, a new goal, then a push for speedy device reviews
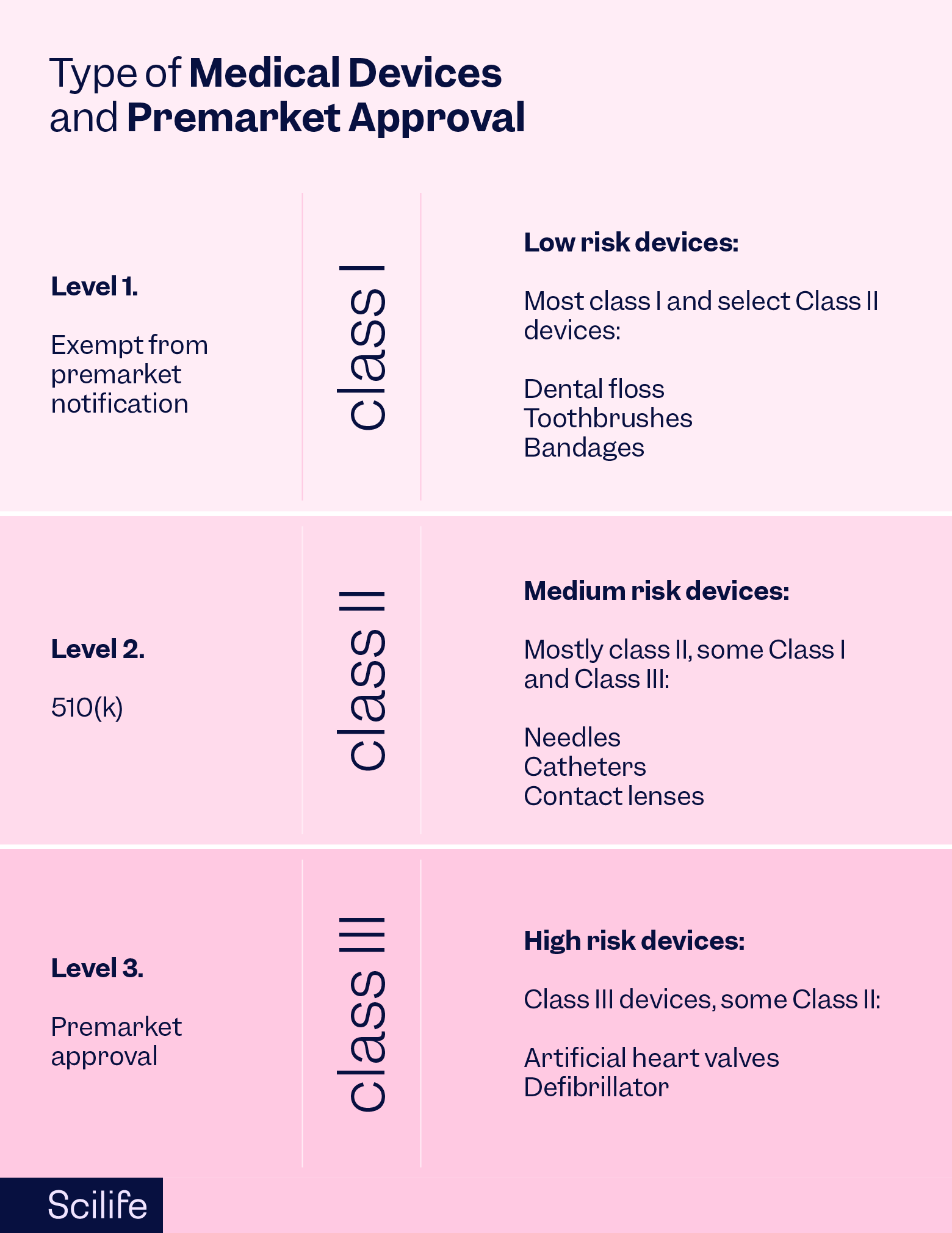
PMA Submissions: Navigating Quality in Premarket Approval
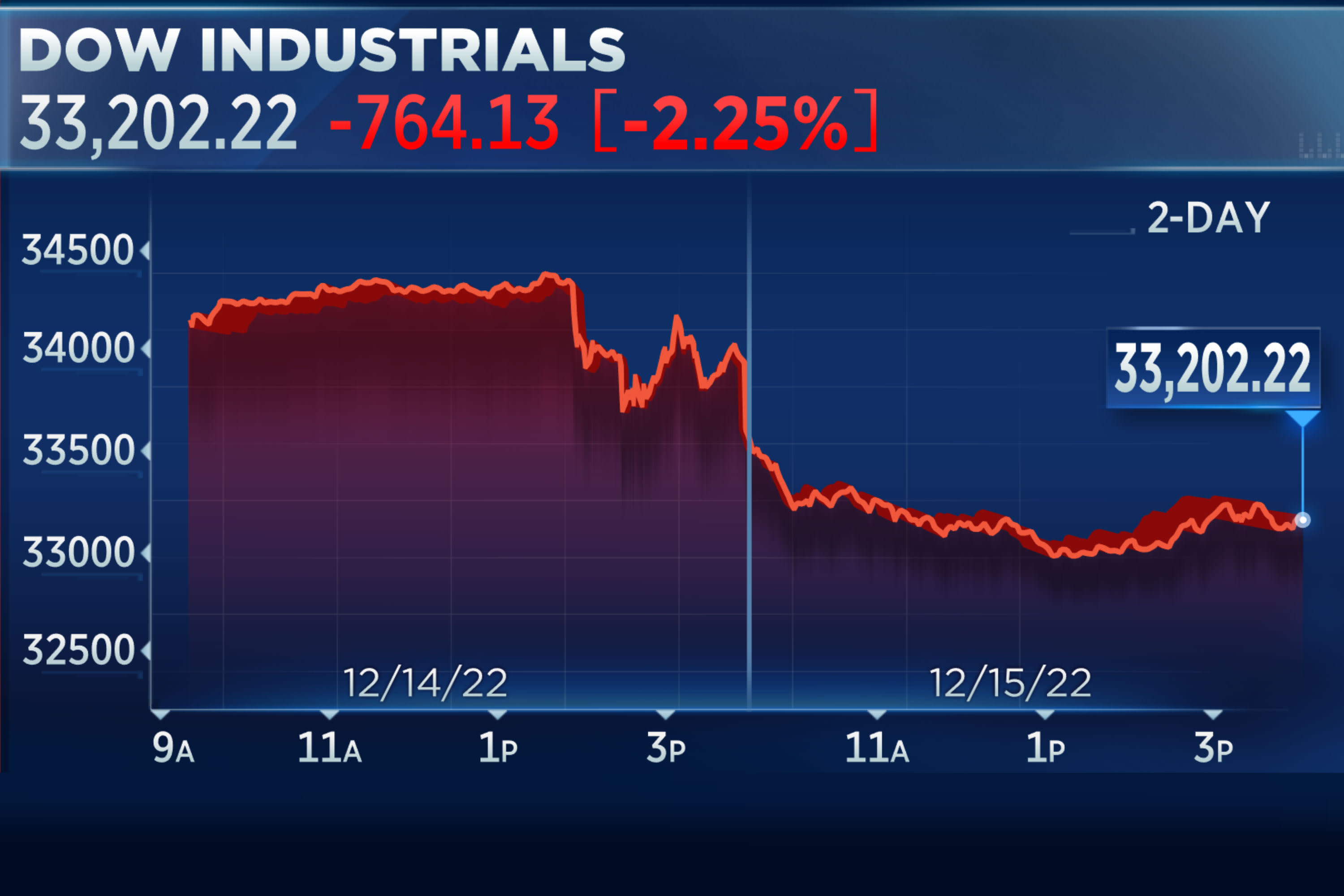
Dow closes out its worst day in three months, falls more than 700 points as recession fears grow

Common Mistakes in Safety Analytics for FDA Submissions

Examining the HHS Proposal for Premarket Notification Exemptions

FDA Releases New Cybersecurity Premarket Guidance

Robert A. Allen, PhD on LinkedIn: #biocompatibility #meddevice #medicaldevice #medicaldevices…
FDA 510(k) Submission: The Anatomy of a Successful Premarket Notification

Understanding Premarket Trading + 5 Tips You Need To Start
Common Mistakes in English and How to Fix Them (PDF)
5 Common Mistakes in Supply Chain Management - 3PL Links
8 Common Manager Mistakes to Avoid with Actionable Processes
5 Common Mistakes You Should Avoid When Conducting A Training Needs Analysis - eLearning Industry
10 Common Mistakes Journalists Make (& How To Avoid Them) - Writers Write
 Nude Vinyl Cutout Triangle Top & Tie Side Thong Swimsuit
Nude Vinyl Cutout Triangle Top & Tie Side Thong Swimsuit Soma 34C Bra Beige Enbliss Luxe Wireless Lace Trim J Hook Back Closure - Helia Beer Co
Soma 34C Bra Beige Enbliss Luxe Wireless Lace Trim J Hook Back Closure - Helia Beer Co Chic Women's Classic Collection Easy-Fit Elastic Waist Pull-On Capri Pant
Chic Women's Classic Collection Easy-Fit Elastic Waist Pull-On Capri Pant Business is busy, but companies still going bust. - The Trades Coach
Business is busy, but companies still going bust. - The Trades Coach Regency History: Kissing under the mistletoe in the Regency
Regency History: Kissing under the mistletoe in the Regency THMO 3 Pair Multipack Womens Winter Leggings Warm Thermal Footless
THMO 3 Pair Multipack Womens Winter Leggings Warm Thermal Footless