At Critical Temperature,pressure and volume . The compressibility
4.6 (287) In stock

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02
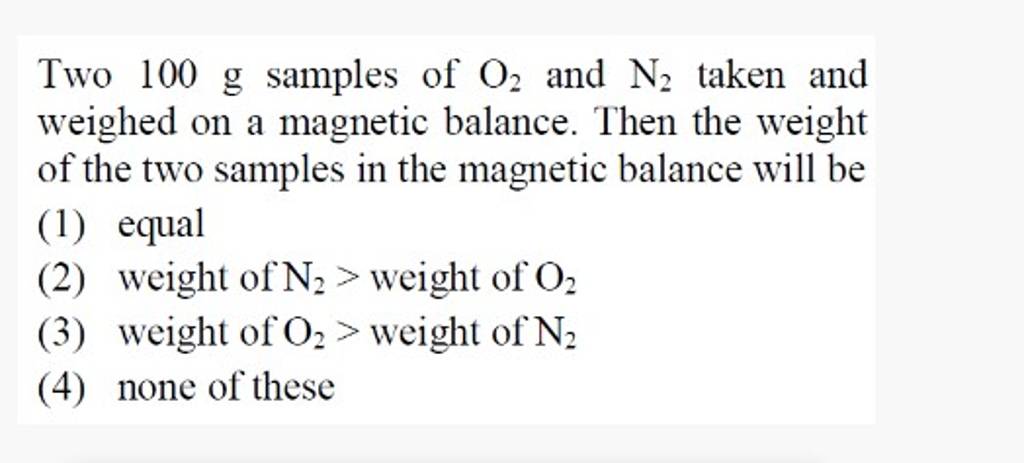
Two 100 g samples of O2 and N2 taken and weighed on a magnetic balance

During osmosis, flow of water through a semipermeable membrane is

States of Matter, PDF, Gases
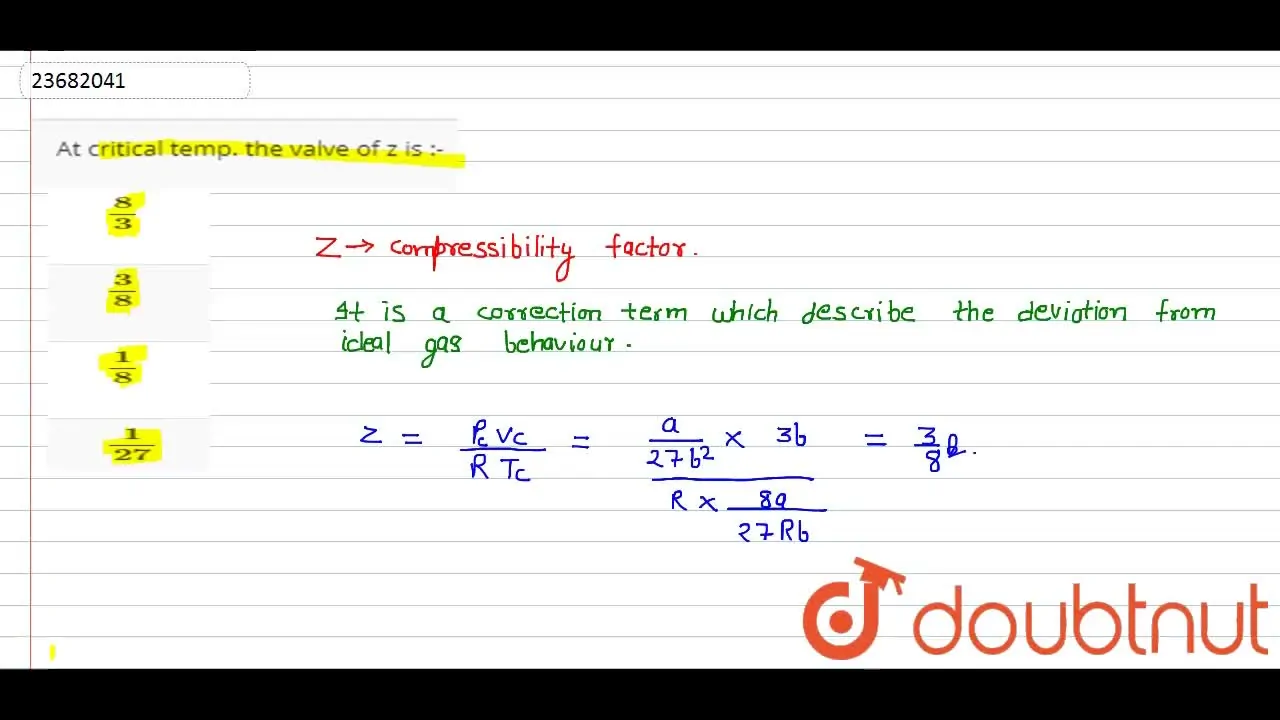
At critical temp. the valve of z is :

LIIS temperature, pressure and volume. The compressibility factor (Z) is 11. At critical temperature. 00 l wo Lliquofration behaviour of tomor
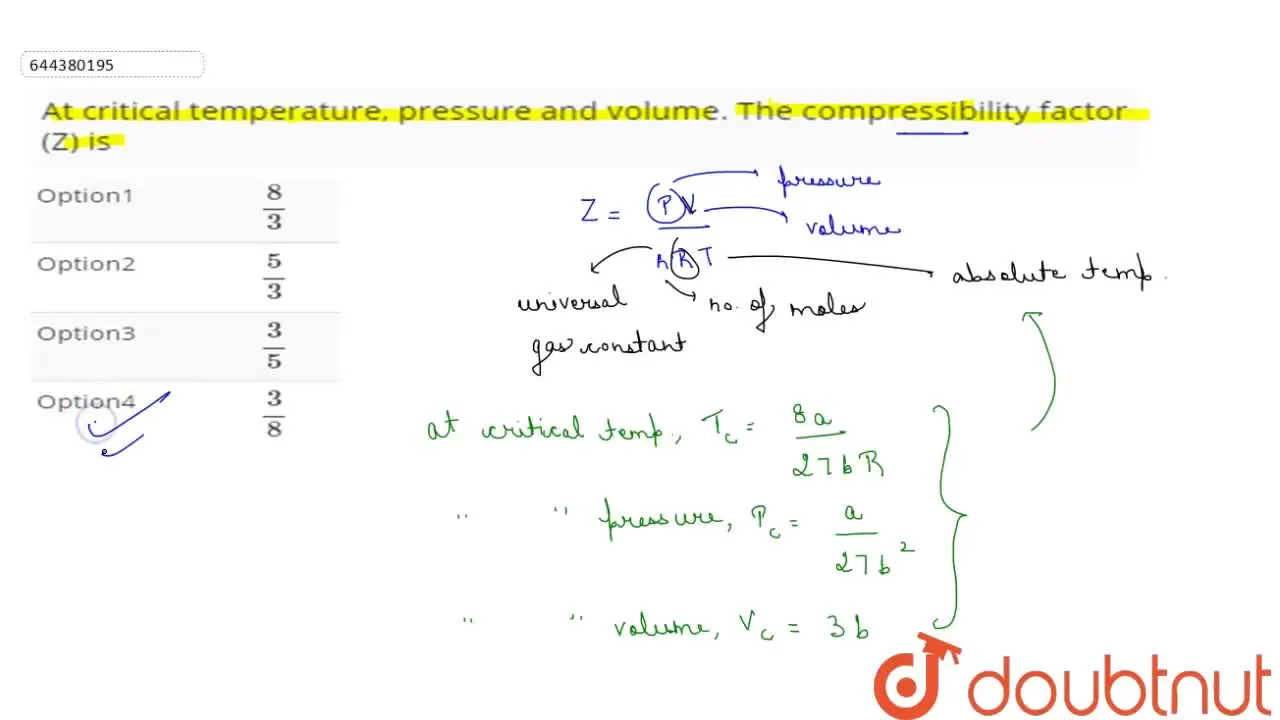
At critical temperature, pressure and volume. The compressibility fact
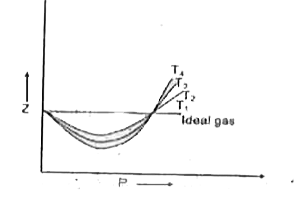
Compressibility factor (Z) is plotted against pressure at different te

States of Matter, PDF, Gases

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02
The compressibility factor a real gas high pressure is:-1 - frac
2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor
 ODM Alluring Elegant Ladies Plus Size Lace Bodysuit Sexy Teddy Lingerie - China Lingerie and Sexy Lingerie price
ODM Alluring Elegant Ladies Plus Size Lace Bodysuit Sexy Teddy Lingerie - China Lingerie and Sexy Lingerie price Lumbar Support Belt Lower Back Brace for Lifting, Herniated Disc
Lumbar Support Belt Lower Back Brace for Lifting, Herniated Disc Super Comfort Bra, Womens Sports Bras Removable Pads Plus Size Sleep Bras For Girls In Yoga Bralette Leisure Stretch Crop Tops Vest
Super Comfort Bra, Womens Sports Bras Removable Pads Plus Size Sleep Bras For Girls In Yoga Bralette Leisure Stretch Crop Tops Vest Knickers Organic Cotton High Waist Full Brief - Navy Ink – Olli Ella USA
Knickers Organic Cotton High Waist Full Brief - Navy Ink – Olli Ella USA Black Saree Shapewear at Rs 190/piece, Saree Shapewear in Surat
Black Saree Shapewear at Rs 190/piece, Saree Shapewear in Surat Delta 9 Gummies 3mg THC and 16mg CBD
Delta 9 Gummies 3mg THC and 16mg CBD