Real Gases. The ideal gas equation of state is not sufficient to
4.6 (421) In stock

Most real gases depart from ideal behaviour at deviation from low temperature high pressure.
High positive potential energy (little separation) Repulsive interactions Intermediate separations attractive interactions dominate Large separations (on the right) the potential energy is zero and there is no interaction between the molecules..
Real gas molecules do attract one another (P id = P obs + constant) Real gas molecules are not point masses (V id = V obs - const.)
V id = V obs - nb b is a constant for different gases P id = P obs + a (n / V) 2 a is also different for different gases Ideal gas Law P id V id = nRT
Critical temperature (T c ) - the temperature above which a gas cannot be liquefied Critical pressure (P c ) – the minimum pressure that needs to be applied at T c to bring about liquefaction
For a perfect gas, the slope is zero Boyle temperature the slope is zero and the gas behaves perfectly over a wider range of conditions than at other temperatures.
Boyle temperature - for a van der Waal s gas, the Boyle temperature (T B ) is written
The reduced state variables are defined
Re-write the Van der Waals in terms of reduced variables
The chemical potential of a real gas is written in terms of its fugacity
In gaseous systems, we relate the fugacity (or activity) to the ideal pressure of the gas via.
Define the fugacity coefficient = f / P For a real gas.
Comparing the chemical potential of the real gas to the chemical potential of an ideal gas at the same pressure
The fugacity coefficients are obtained from the compression factors (Z) as shown below

Real Gases Introductory Chemistry

Solved 4. Real gas effects can be expressed as departures

Graham's Law of Diffusion vs. Effusion

PPT - Chemistry 231 PowerPoint Presentation, free download - ID:917796
GASES. - ppt download

Ideal vs. Real Gas Laws Differences, Formula & Assumptions
6.3: Combining the Gas Laws: The Ideal Gas Equation and the
Density-wave ordering in a unitary Fermi gas with photon-mediated

Libertarianism, Climate Change, and Individual Responsibility

Prepared By: Bhadka Ravi H. Guided By: Mr. P. L. Koradiya - ppt download
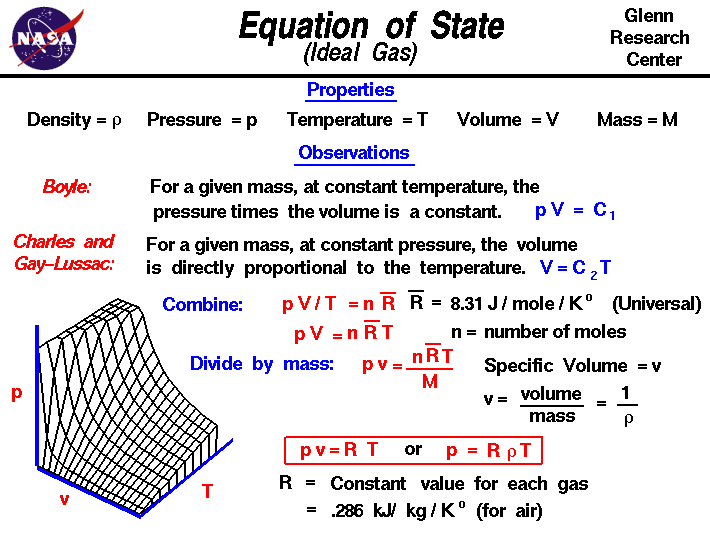
Equation of State

Relief Valve Sizing Guide on the Phase Envelope
PPT - Chemistry 231 PowerPoint Presentation, free download - ID:1431197

Deviations from the Ideal Gas Law and Chemistry in the Atmosphere Chemistry 142 B Autumn Quarter, 2004 J. B. Callis, Instructor Lecture # ppt download
Solved As a first approximation, the compression factor, Z
At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{
 Lands' End Women's Starfish Mid Rise Knit Leggings
Lands' End Women's Starfish Mid Rise Knit Leggings V-LINE Mask Pack for Double Chin / Extra V-Line Lift Up – Chaga Beauty
V-LINE Mask Pack for Double Chin / Extra V-Line Lift Up – Chaga Beauty EHQJNJ Strapless Bra Push up Plus Size Womens One Word Wrap Chest Lace Underwear Womens No Steel Ring No Cup Anti Slip and Anti Slip Belt Ining No
EHQJNJ Strapless Bra Push up Plus Size Womens One Word Wrap Chest Lace Underwear Womens No Steel Ring No Cup Anti Slip and Anti Slip Belt Ining No OnGossamer Women's Racy Lace Hip G Thong Panty
OnGossamer Women's Racy Lace Hip G Thong Panty 2023 Pantalones De Terciopelo Paras Mujer Leggings De Invierno Para Nieve Frio
2023 Pantalones De Terciopelo Paras Mujer Leggings De Invierno Para Nieve Frio Review: OPPO A98 5G, an affordable phone that looks great in a
Review: OPPO A98 5G, an affordable phone that looks great in a