The role of the compressibility factor Z in describing the volumetric behavior of gases
5 (581) In stock
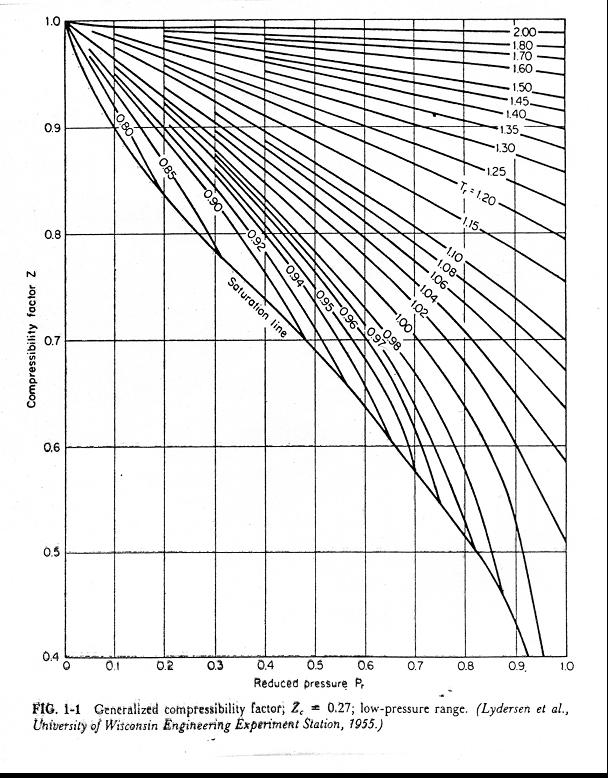
In this post I will give a recapitulation of the role the compressibilty factor Z plays in the volumetric behavior of gases. The purpose of this post is also to give some background to the first post of June , 2013 describing a compact, explicit equation for the vapor compressibility factor Z in the sub-critical…

Gas compressibility factor Z: Ideal gas vs Real gas

SOLVED: What is the physical significance of the compressibility factor Z ?

Compressibility Factor - an overview

PDF) New correlations to predict natural gas viscosity and compressibility factor

Compressibility factor (gases) - Citizendium

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Compressibility factor (z): real gases deviate from ideal behav-Turito

Gas Compressibility - an overview

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

The Compression Factor, Z, and Real Gases - What you NEED to Know!

Acentric Factor - an overview
physical chemistry - Why do some gases have lower value of Z for a
Consider the graph between compressibility factor Z and pressure P
What is the value of compressibility factor in terms of vander
- LoL Esports (@lolesports) • Instagram photos and videos
- Aerie Satin Stretch High Waisted Boybrief Underwear
 Buy Adidas Pacer 3-Stripes Woven Two-in-One Shorts black/white
Buy Adidas Pacer 3-Stripes Woven Two-in-One Shorts black/white LAPASA Mens Pajama Set 100% Cotton Flannel Top Long Sleeve & Bottom Pants Plaid Sleepwear PJ Sleepwear Lounge Comfy Button-Down M95 Large
LAPASA Mens Pajama Set 100% Cotton Flannel Top Long Sleeve & Bottom Pants Plaid Sleepwear PJ Sleepwear Lounge Comfy Button-Down M95 Large Bust of Woman Art Nouveau Sculpture 12, Female Bust Statue Art, Women Girl Concrete Home Ornament, Bust Head and Shoulders of Young Woman
Bust of Woman Art Nouveau Sculpture 12, Female Bust Statue Art, Women Girl Concrete Home Ornament, Bust Head and Shoulders of Young Woman Seamless abdominal binder - RECOVA®
Seamless abdominal binder - RECOVA® MU DAILY NON WIRED BRA CUP B / LADIES BRA 226 /BAJU DALAM WANITA / HIGH QUALITY SLEEP
MU DAILY NON WIRED BRA CUP B / LADIES BRA 226 /BAJU DALAM WANITA / HIGH QUALITY SLEEP Men's ROCKSTAR Sushi - JAX Biker Pants in Black Twill – J. Ransom LA
Men's ROCKSTAR Sushi - JAX Biker Pants in Black Twill – J. Ransom LA Soma and the Sacred Feminine: Reflections from Ancient Indian Myth - Chacruna
Soma and the Sacred Feminine: Reflections from Ancient Indian Myth - Chacruna Quote: If you want to stand out, don't - CoolNSmart
Quote: If you want to stand out, don't - CoolNSmart- 6 x Womens Bonds Maternity Bumps Bikini Underwear Undies Black


