Developing a Thermodynamical Method for Prediction of Activity
4.7 (194) In stock

Results of the experimental measurements on the partial molar volume of kerosene used as a medium for dissolving TBP are utilized to determine the activity of TBP in the binary kerosene-TBP solution through the application of Gibbs-Duhem equation. The treatment is based on combination of the experimental data with the thermodynamic values available on the compressibility factor of pure kerosene at room temperature. It is shown that the activity of TBP in kerosene has a positive deviation from ideality with an activity coefficient derived as follows:1) at X TBP ≤ 0.01: γ TBP = 42.530, 2) at the 0.01 X TBP 0.2: 3) at the higher TBP concentrations 0.2 X TBP 0.97: and 4) at TBP Raoultian concentrations 0.97 ≤ X TBP:γ TBP = 1. These quantities can be utilized at temperature closed to 298 K.

E. ALAMDARI, Professor (Associate), PhD, Amirkabir University of Technology, Tehran, TUS, Department of Mining and Metallurgical Engineering

Separation of Re and Mo from Roasting-Dust Leach-Liquor Using Solvent Extraction Technique by TBP
Development of an Experimental Data Base and Theories for Prediction of Thermodynamic Properties of Aqueous Electrolytes and Nonelectrolytes of Geochemical Significance at Supercritical Temperatures and Pressures. - UNT Digital Library

PDF) The performance of UNIFAC and related group contribution models Part I. Prediction of infinite dilution activity coefficients

Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

E. ALAMDARI, Professor (Associate), PhD, Amirkabir University of Technology, Tehran, TUS, Department of Mining and Metallurgical Engineering
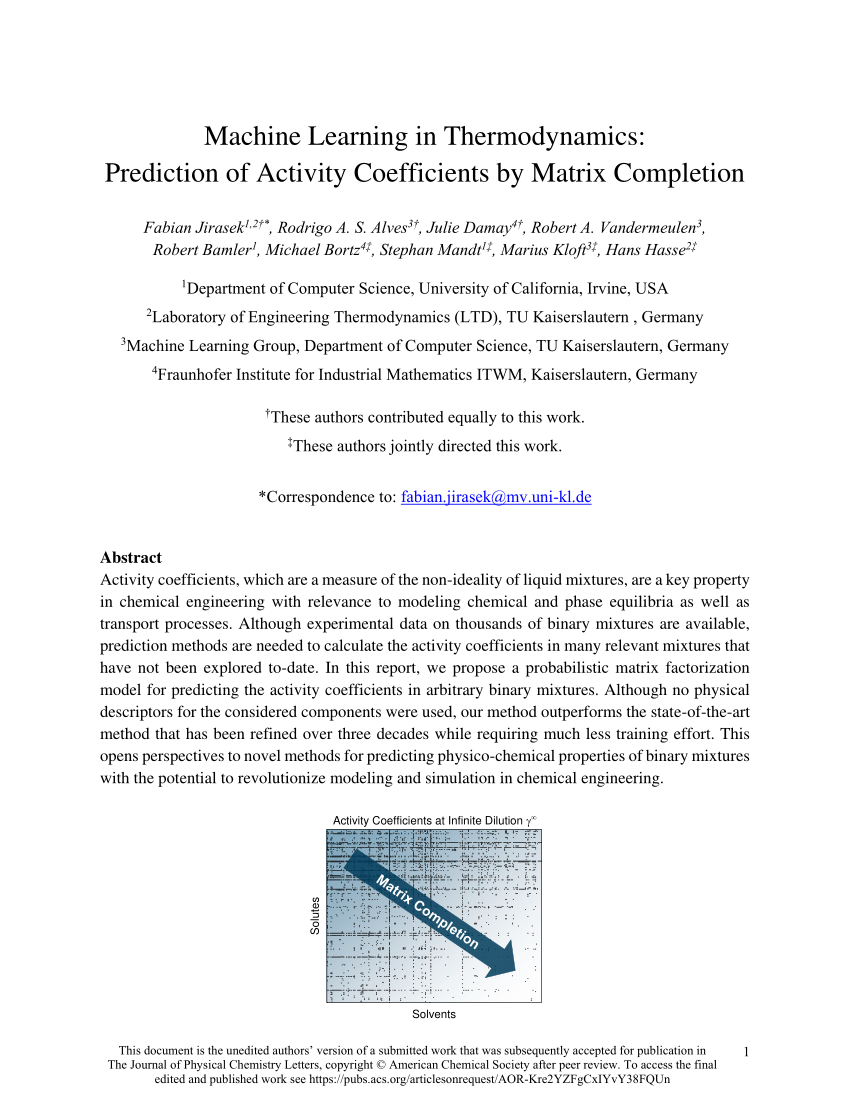
PDF) Machine Learning in Thermodynamics: Prediction of Activity Coefficients by Matrix Completion

PHREEQC > PHREEQC Basic

Synergistic effect of MEHPA on co-extraction of zinc and cadmium with DEHPA
Frontiers Novel Alloy Design Concepts Enabling Enhanced Mechanical Properties of High Entropy Alloys

Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

PDF) Thermodynamics of extraction of Zn2+ from sulfuric acid media with a mixture of DEHPA and MEHPA
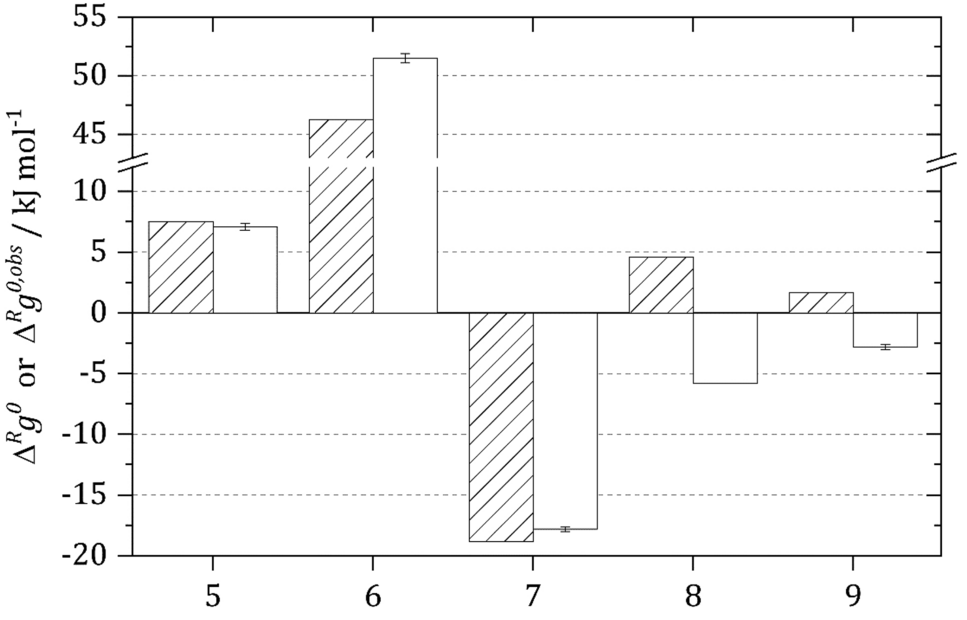
New thermodynamic activity-based approach allows predicting the feasibility of glycolysis
Solved Show that the compressibility factor of van der Waals
For H(2) gas, the compressibility factor,Z = PV //n RT is
 Utilitech 50-ft 12/3-Prong Outdoor Sjtw Heavy Duty Lighted
Utilitech 50-ft 12/3-Prong Outdoor Sjtw Heavy Duty Lighted Fantastiques vêtements outdoor
Fantastiques vêtements outdoor Buy Women's Wirefree Padded Super Combed Cotton Elastane Stretch
Buy Women's Wirefree Padded Super Combed Cotton Elastane Stretch Couples Matching Underwear Set Women Plus Size Underwire Lingerie
Couples Matching Underwear Set Women Plus Size Underwire Lingerie Rajee Rajindra Narin on X: CAST YOUR VOTE! Vote #1 for me to have a breast reduction. Vote #2 for me to have a booty reduction. Vote #3 for me to have
Rajee Rajindra Narin on X: CAST YOUR VOTE! Vote #1 for me to have a breast reduction. Vote #2 for me to have a booty reduction. Vote #3 for me to have Cacique cotton no-wire full - Gem
Cacique cotton no-wire full - Gem