Understanding the Insulin Pump Recall
4.7 (591) In stock
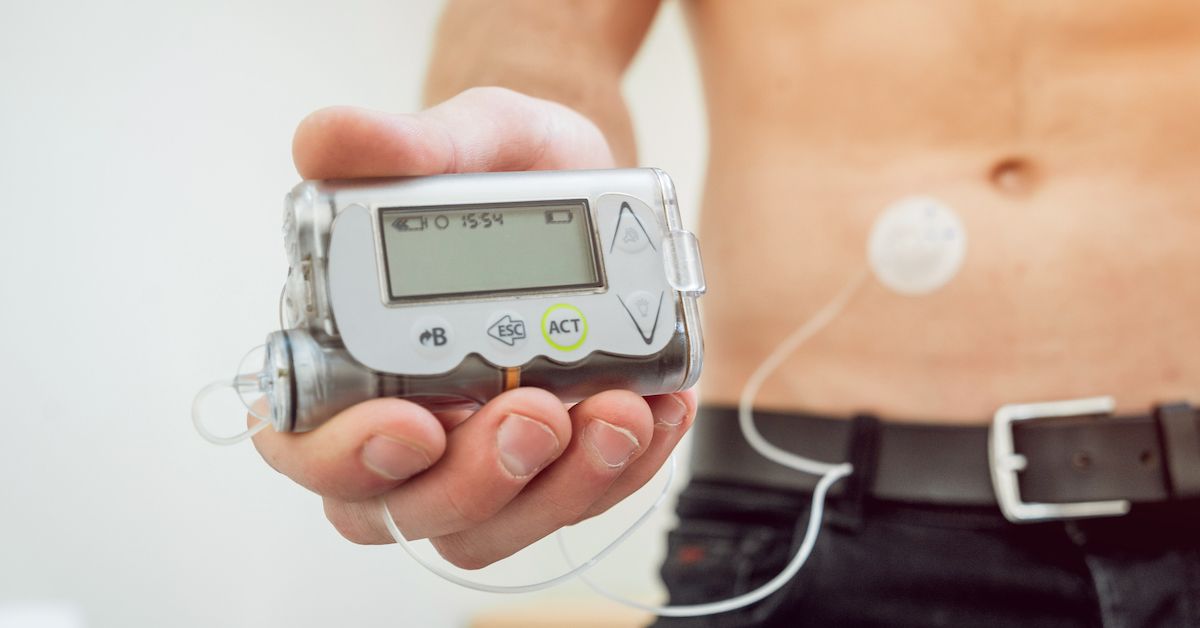
Medtronic is recalling defective MiniMed insulin pumps that could cause severe hypoglycemia or hyperglycemia resulting in seizures, coma and even death

Medtronic MiniMed Insulin Pump Recalls - The Lake Law Firm

Medtronic Insulin Pumps - DeGaris Law, LLC

Medtronic MiniMed Insulin Pump Lawsuit – RolsHite Tort Law

Compare Medtronic Insulin Pump Systems

Medtronic MiniMed Insulin Pump Lawsuit - Seeger Weiss LLP
Medtronic recalls certain MiniMed insulin pumps tied to 1 death

Insulin Pump Recalls
MiniMed™ 780G System

FDA Issues Class I Recall of Certain Medtronic Insulin Pumps
Class 2 Recall Issued for Nearly 2,000 Medtronic MiniMed Insulin Pumps – Parker Waichman LLP

Insulet has a Class I recall for the Omnipod 5 Android App
Regulators recall a Medtronic product over insulin worries
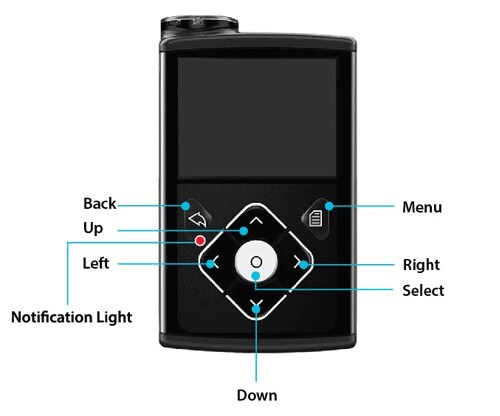
Pump Buttons - MiniMed™ 630G System Support

Safety alerts Medtronic Diabetes
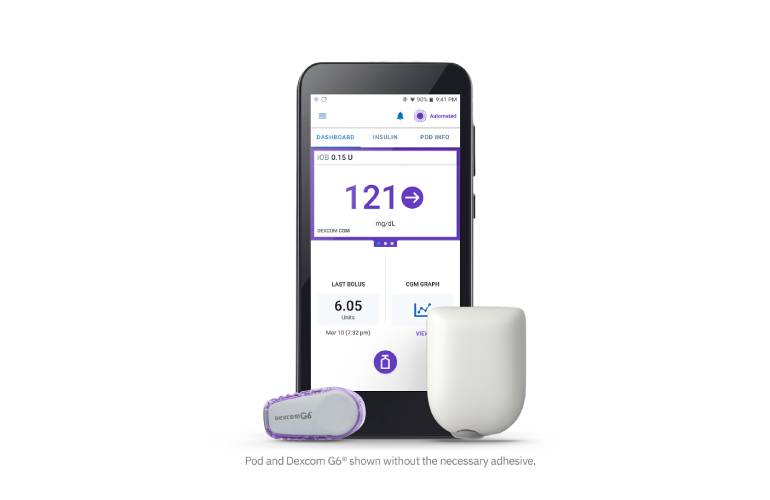
Recalls Archives - Page 3 of 9 - Drug Delivery Business
Type 1 Diabetes, Insulin Pump Therapy
insulin pump diagram – NIH Director's Blog
Insulin Pump Vulnerable to Hacking, Johnson & Johnson Warns