physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
4.8 (739) In stock

In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

Effects of Microporosity and Surface Chemistry on Separation

822 questions with answers in PHYSICAL CHEMISTRY

Minerals, Free Full-Text
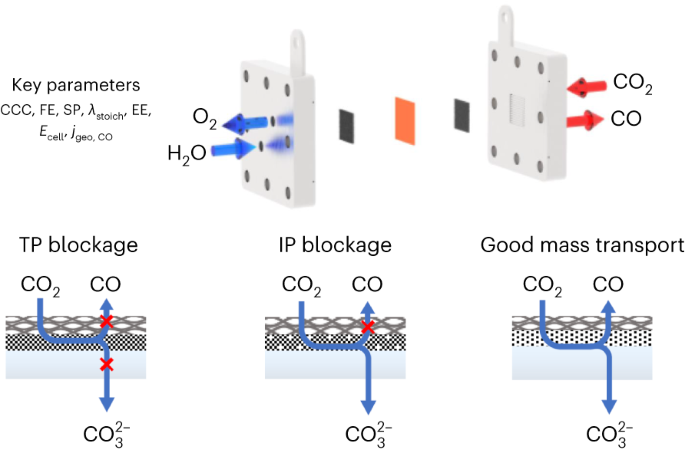
Design and diagnosis of high-performance CO2-to-CO electrolyzer
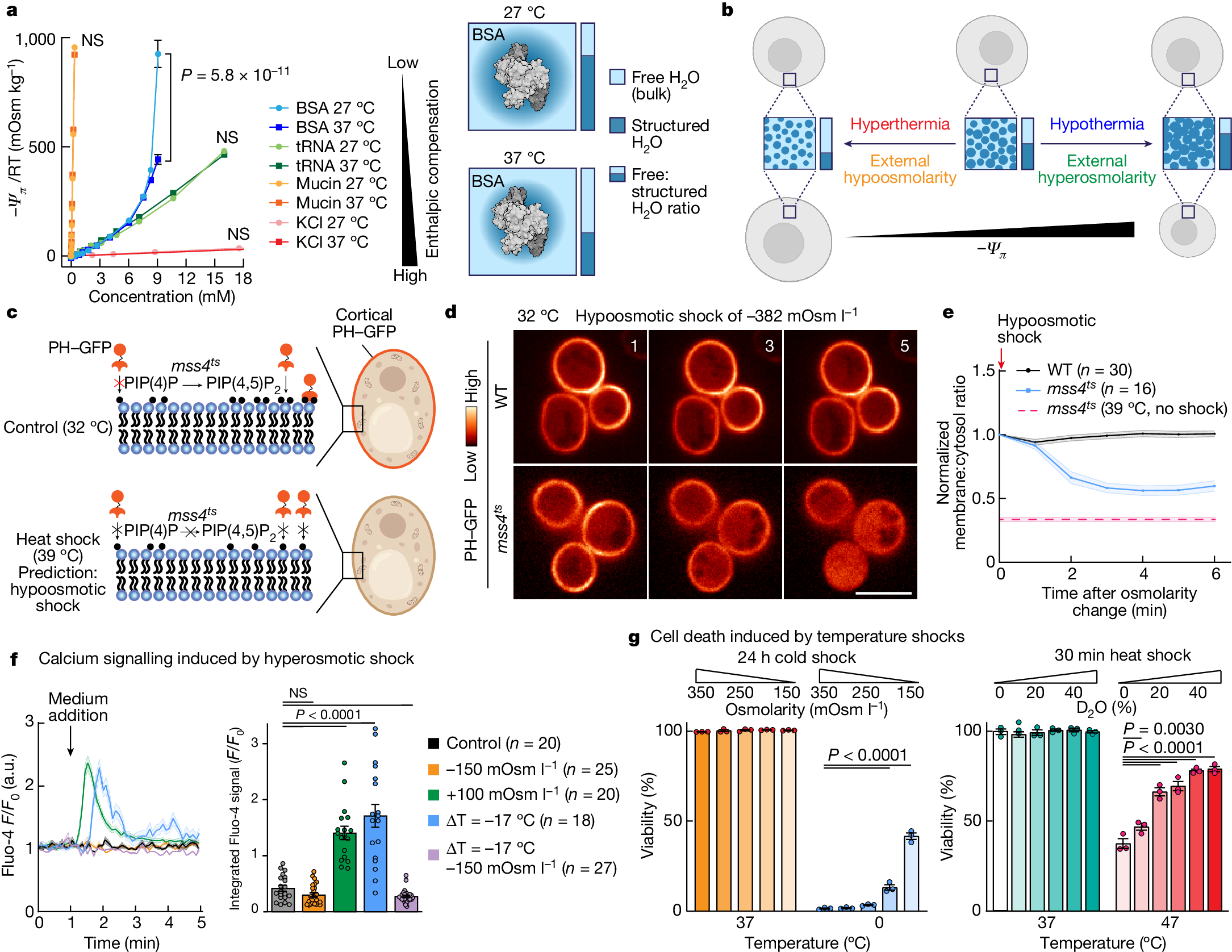
Macromolecular condensation buffers intracellular water potential
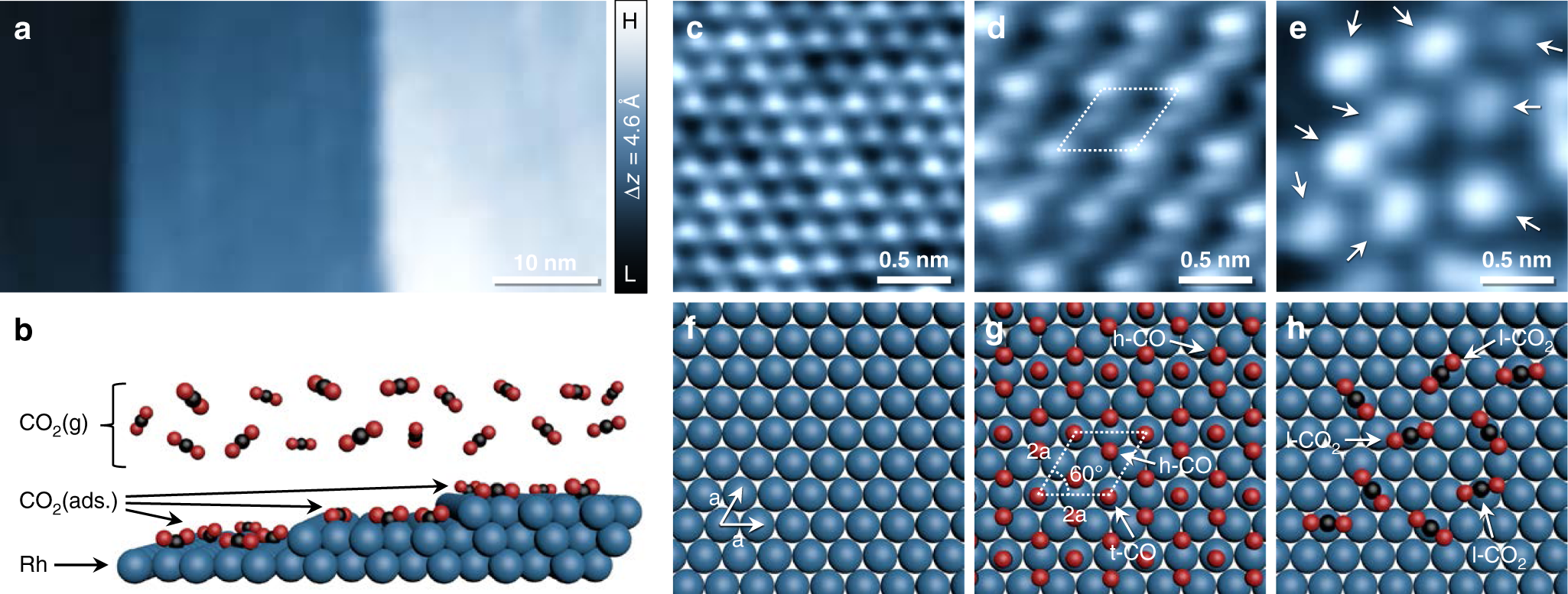
How Rh surface breaks CO2 molecules under ambient pressure

Gas - Wikipedia
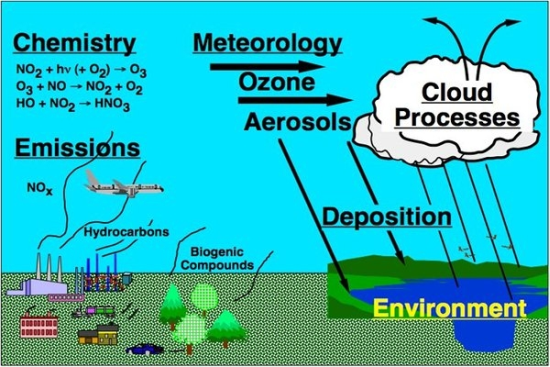
Atmosphere, Free Full-Text

Behavior of Gases

Water Phase Diagram, Comparisons & Importance - Lesson
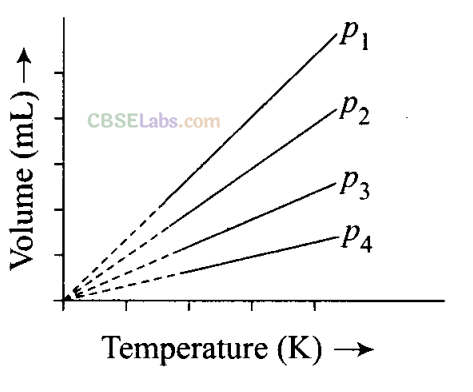
NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter
Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
Compressibility Factor Z Important Concepts and Tips for JEE Main
Compressibility Factor Charts - Wolfram Demonstrations Project
Real gasses For an ideal gas, the compressibility factor Z = PV
Compressibility factor Z as function of temperature T with lines of
 Buy Fancy Fila Sports Shoes Online for Men in India
Buy Fancy Fila Sports Shoes Online for Men in India- Bloch Amsterdam - Work out in the Bloch® Balance Fitness shoes. This innovative range of Barre and Pilates shoes are technically advanced having multiple patents pending. These slip-on and toeless studio shoes
 Sculpted By Aimee
Sculpted By Aimee Premium Colombian Shapewear Faja Shapewear Man Thermal Tank Top. Body Briefers For Men Slimmer Clothing
Premium Colombian Shapewear Faja Shapewear Man Thermal Tank Top. Body Briefers For Men Slimmer Clothing Lou & Grey Pink Floral Leggings Size Medium.
Lou & Grey Pink Floral Leggings Size Medium. Summer Jumpsuits for Women Trendy Flounce Top Off Shoulder
Summer Jumpsuits for Women Trendy Flounce Top Off Shoulder
