Consider the graph between compressibility factor Z and pressure P The correct increasing order of ease of liquefaction of the gases shown in the above graph is
4.8 (324) In stock

Z1 means force of attraction dominating ie a is considerable b can be negligible at low temperature and low pressure Lower is the value of Z easier is the process of liquification
The compressibility factor is actually a factor that corrects the actual value of the gas versus the ideal gas. Let us learn and understand this concept.
Watch this video to understand the behaviour of real gases with the help of the compressibility factor. This is an important topic for JEE main.
What is the compressibility factor, and how does it vary with an increase in temperature and pressure? Watch this video to get the answer. This is an importa
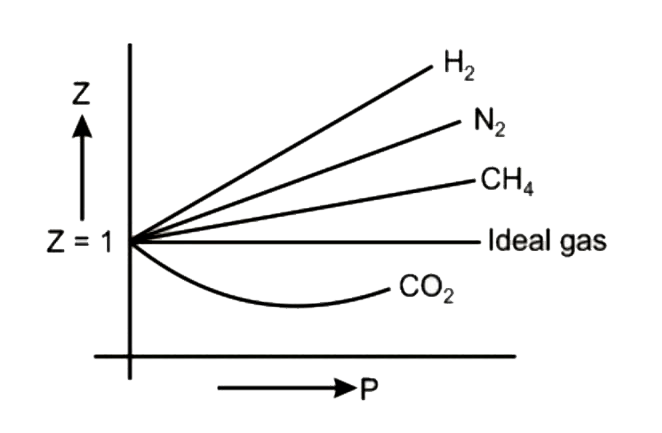
Consider the graph between compressibility factor Z and pressure P

Liquefaction of Gases - GeeksforGeeks

The given graph represents the variation of Z (compressibility

Compressibility Factor - an overview

Doc 117 b p s xi chemistry iit jee advanced study package 2014 15 by S.Dharmaraj - Issuu

Compressibility factor - Wikipedia
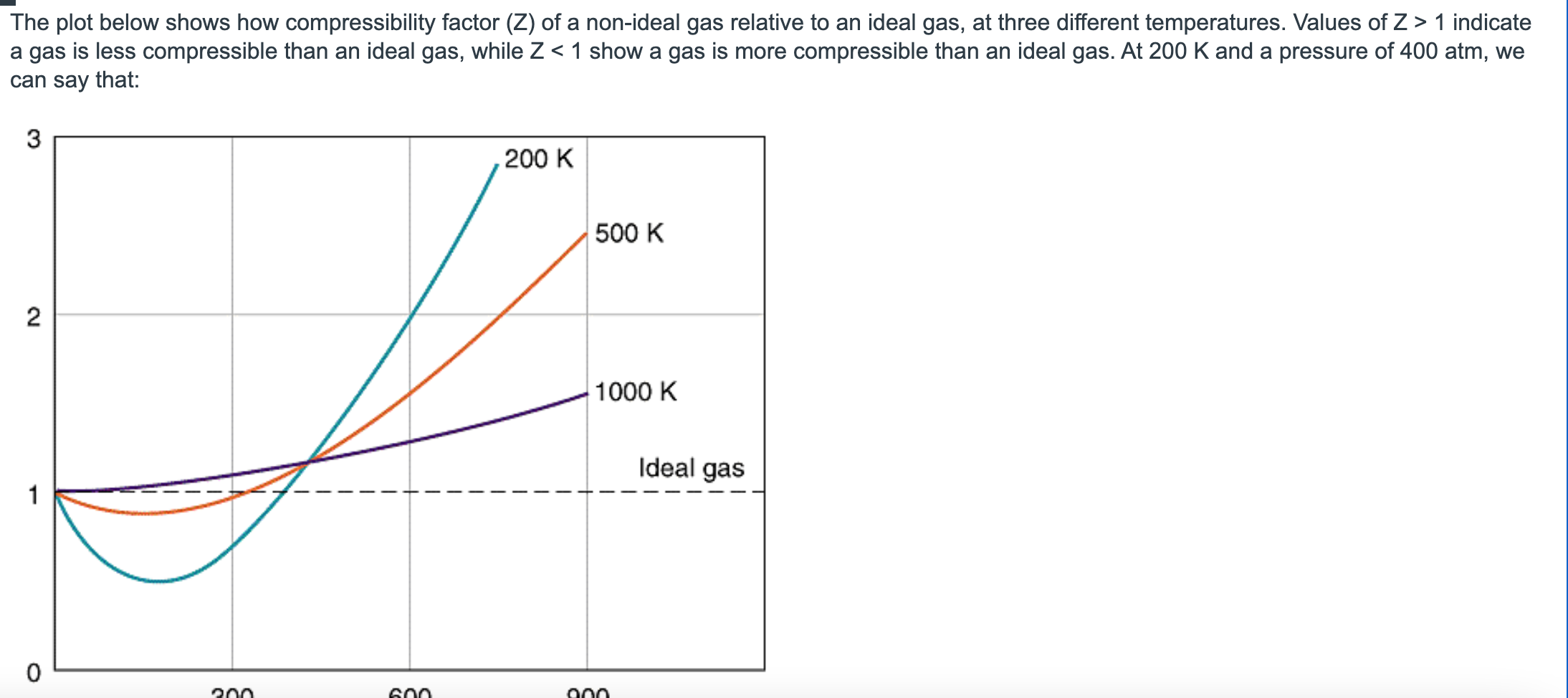
Solved The plot below shows how compressibility factor (Z)

Consider the graph between compressibility factor Z and pressure P. The correct increasing order of ease of liquefaction of the gases shown in the above graph is
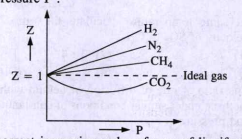
Consider a graph between compressibility factor Z and pressure P

The given graph represents the variation of Z(compressibility
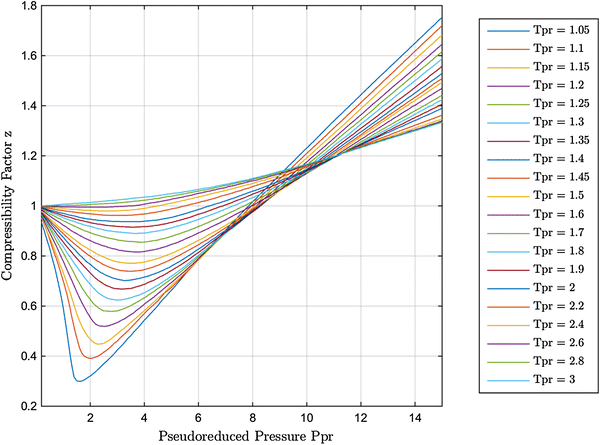
New explicit correlation for the compressibility factor of natural

Compressibility factor (z): real gases deviate from ideal behav-Turito
Summary of Equations used to evaluate compressibility factor, z
physical chemistry - Why do some gases have lower value of Z for a
The compressibility factor Z a low-pressure range of all gases
 Seamless Support Wireless Comfort Bra - 3pc - Breathable Mesh Design, Anti-Chafing, Removable Pads, & Versatile Stretch Sports Freedom Bra - Lavender
Seamless Support Wireless Comfort Bra - 3pc - Breathable Mesh Design, Anti-Chafing, Removable Pads, & Versatile Stretch Sports Freedom Bra - Lavender Chantelle Women's Basic Invisible Smooth T-Shirt Bra , Rose , 32E
Chantelle Women's Basic Invisible Smooth T-Shirt Bra , Rose , 32E Leggings Efecto Piel Brillantes - México
Leggings Efecto Piel Brillantes - México CUUP 38G The Plunge Slate Bra
CUUP 38G The Plunge Slate Bra Kit 5pçs Cueca Calvin Klein Underwear Pride Azul/Rosa - Compre
Kit 5pçs Cueca Calvin Klein Underwear Pride Azul/Rosa - Compre leggings Deportivas Ropa Deportiva De Moda Licras Pantalones Yoga
leggings Deportivas Ropa Deportiva De Moda Licras Pantalones Yoga