Compressibility factor (Z) for a van der Waals real gas at
4.6 (266) In stock

Share your videos with friends, family and the world

Compressibility factor Z - Gaseous State
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma

6.3: Van der Waals and Other Gases - Physics LibreTexts

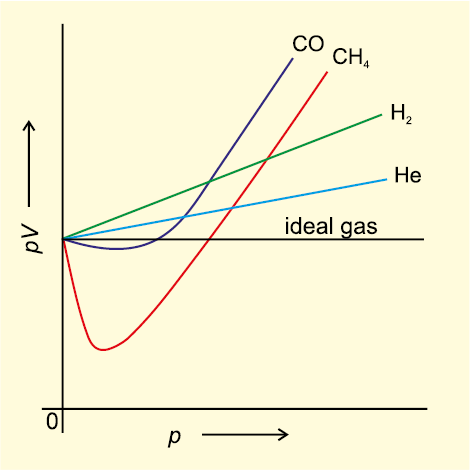
Explain how the compression factor varies with pressure and
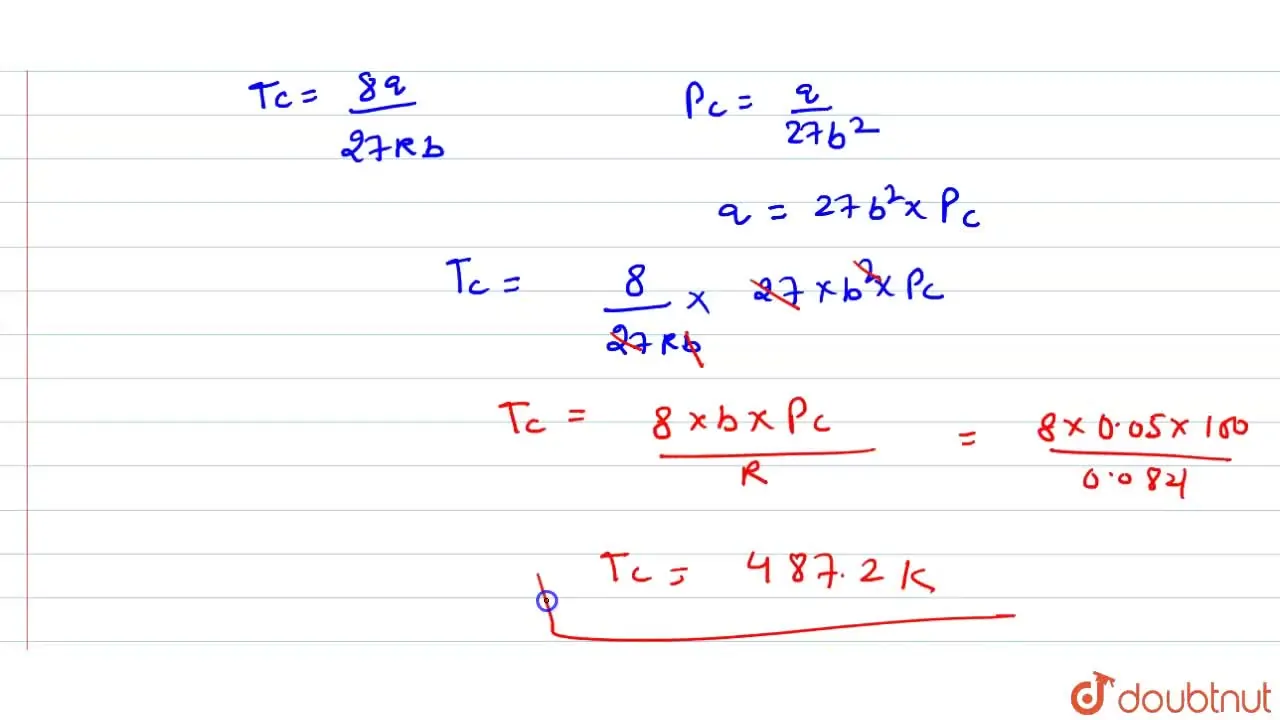
Calculate the critical temperature of a Van der Waals gas for which p(

Solved RT B 2. The compressiblity factor for a gas is

The compressibility factor Z of one mole of Vander Waals gas with negligible 'a' value is a) bp/RT b) [1-(bp/RT) c)[1 (bp/RT) d) (1/bp)? - EduRev NEET Question

Non Ideal Gas Behavior-chemistry - Non Ideal Gas Behavior Calculate the compressibility factor (Z) - Studocu

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
Sections

The compression factor (compressibility factor) for 1 mol of a van der
New explicit correlation for the compressibility factor of natural
Gas compressibility factor Z: Ideal gas vs Real gas
Compressibility Factor (Z) And Pressure Bar Royalty Free SVG
Calculate the Compressibility Factor 'z' for Hydrocarbon Gases
How the ideal gas law helped us creating a software tool called





