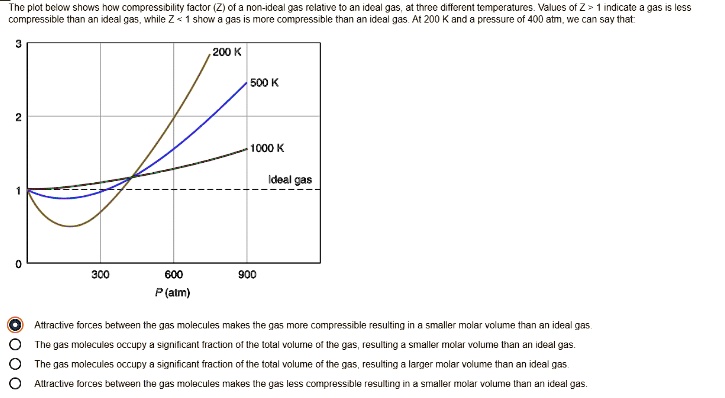
SOLVED: Plot bclon shcs now compressibility factor (Ziofa non-Idc? 935 relative to an ideal gas; J1 force differential Mocraiurc: Values of Z indicate compressibility and inan re any more compressible ideal gas
4.6 (791) In stock
VIDEO ANSWER: We are going to see the difference between the liquid and the guest. The chemical entities are associated with bonds in gas. There are chemical entities away from each other. The guests here have a random motion system that is
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

SOLVED: Plot bclon shcs now compressibility factor (Ziofa non-Idc? 935 relative to an ideal gas; J1 force differential Mocraiurc: Values of Z indicate compressibility and inan re any more compressible ideal gas

Non-Ideal Gas Behavior Chemistry: Atoms First

What is the value of compressibility factor for a non-ideal gas? - Quora

ideal gas - Compressibility factor and deviation from ideality - Chemistry Stack Exchange

What is the value of compressibility factor for a non-ideal gas? - Quora

Compressibility factor - Wikipedia

Solved The graph of compressibility factor (Z)v/sP for 1 mol

Compressibility Factor - an overview

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following
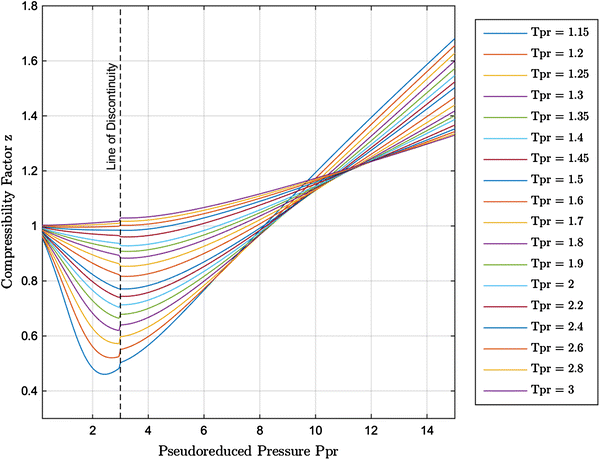
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

Statement-1. Compressibility factor of non-ideal gases is always less than1. Statement-2. Non-id

Non-Ideal Gas Behavior Chemistry: Atoms First

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Statement-1 is correct, Statement-2 is incorrect.
At Critical Temperature,pressure and volume . The compressibility
Gas Compressibility - an overview
Compressibility Chart - an overview
 Bisley WOMEN'S X AIRFLOW RIPSTOP SHIRT Online Australia
Bisley WOMEN'S X AIRFLOW RIPSTOP SHIRT Online Australia Up Flare Leggings Navy Blue (Custom-Made)
Up Flare Leggings Navy Blue (Custom-Made) Classic Walnut, 1 3/8 Strap, Dress Belt
Classic Walnut, 1 3/8 Strap, Dress Belt Incredible Spring 2007 John Galliano Strapless Black Lace Dress w
Incredible Spring 2007 John Galliano Strapless Black Lace Dress w- Lovies Premium Pull-Up Pants Size 3 Nappies 40 Pack, Potty Training & Pull Up Nappies, Nappies, Baby
 Wacoal Women's Plus-Size Back Appeal Underwire Bra Bra, Black, 32D : : Fashion
Wacoal Women's Plus-Size Back Appeal Underwire Bra Bra, Black, 32D : : Fashion