For a given gas, a graph is shown between compressibility factor
4.7 (703) In stock

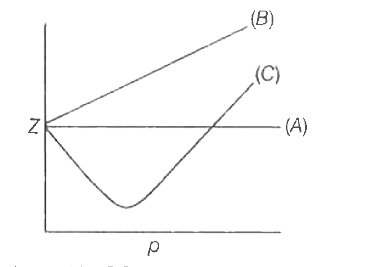
By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.
Telugu] The variation of compressibility factor (Z) with pressure (p

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

Non-Ideal Gas Behavior Chemistry: Atoms First
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora

gas laws - How to find the temperature relationship between the isotherms in a compressibility factor (Z) vs pressure graph? - Chemistry Stack Exchange

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.

Deviation Of Real Gas From Ideal Gas Behavior

Compressibility factor (Z) is plotted against pressure at different te

Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a
New explicit correlation for the compressibility factor of natural
Compressibility factor Z as function of temperature T with lines
The compressibility factor Z a low-pressure range of all gases
Consider the graph between compressibility factor Z and pressure P





