For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may
5 (425) In stock


The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.

Compressibility factor - Wikipedia

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.

Temperature and Pressure Measurements of an Ideal Gas - Because the ideal gas was in a closed - Studocu

1.7: Connecting the van der Waals and the viral equations- the Boyle temperature - Chemistry LibreTexts

A graph Z vs P is plotted N_2 gas different temperatureThe correct relationship between temperatures
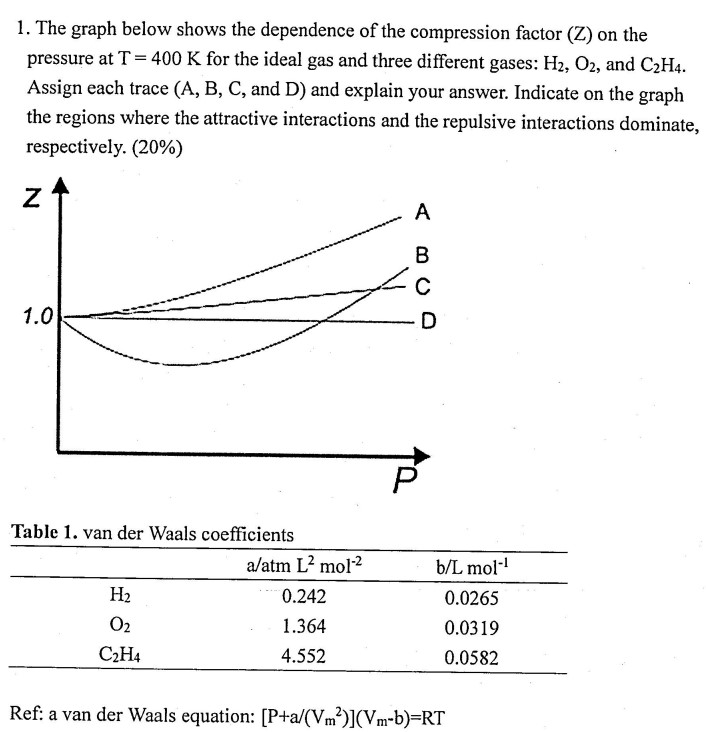
Solved The graph below shows the dependence of the

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

compressibility Factor v/s Pressure/ Temperature Graph . States of Matter Class XI.
Physical Chemistry The Compression Factor (Z) [w/1 example]
Solved F The compressibility factor ( Z ) of liquid faca
Determine Compressibility of Gases
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT
 Rubber Pants with Dots - KinkyDiapers
Rubber Pants with Dots - KinkyDiapers Boho Two Piece Two Piece Wedding Dress 2021 Crop Top Chiffon, Long Sleeve, Floor Length, Perfect For Summer Beach Parties And Bohemian Country Bridal Gowns Vestio De Novias From Bridalstore, $81.41
Boho Two Piece Two Piece Wedding Dress 2021 Crop Top Chiffon, Long Sleeve, Floor Length, Perfect For Summer Beach Parties And Bohemian Country Bridal Gowns Vestio De Novias From Bridalstore, $81.41 Top 5 Running Injuries: Iliotibial Band Syndrome
Top 5 Running Injuries: Iliotibial Band Syndrome Rated Best Indoor/Outdoor Putting Green- Golf Putting Mat- PrimePutt Pro Turf Best Putting Greens
Rated Best Indoor/Outdoor Putting Green- Golf Putting Mat- PrimePutt Pro Turf Best Putting Greens How to Find an Art Class near You? Art Canina Might Be the Solution
How to Find an Art Class near You? Art Canina Might Be the Solution- Ann Summers Understated padded balcony bra with delicate lace overlay in red
