Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
4.7 (407) In stock

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
Which gas shows the maximum deviation from ideal gas, CO2 or NH3

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i
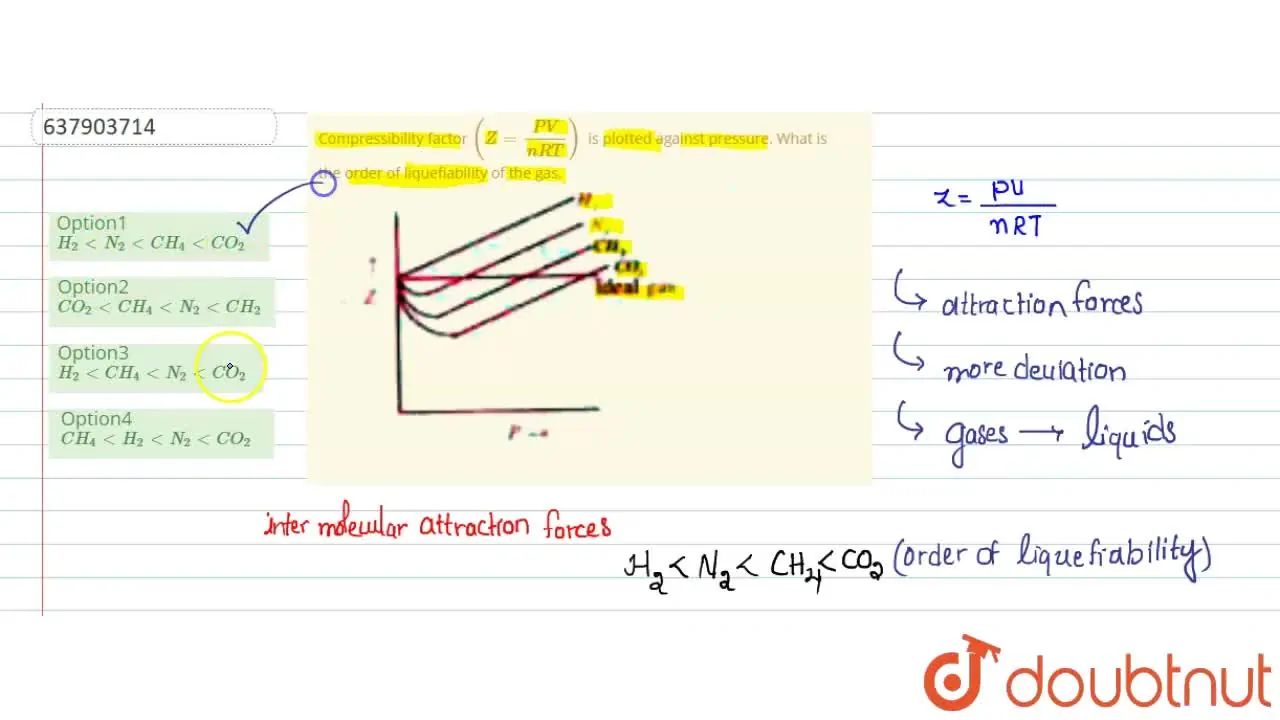
Telugu] Compressibility factor (Z = (PV)/(nRT)) is plotted against p

Compressibility Factor of Gas Overview, Equation & Chart
Chapter 3 - Physical Properties of Fluids: Gas Compressibility

Compressibility Factor - an overview
How do we sketch pressure against pV/T where p is pressure, V is

gaseous state
Compressibility factor - Wikipedia
Virial coefficients: empirical approx. of the compression factor
Solved 3.91. The definition of compressibility factor Z, Eq
Compressibility factor z versus 100/V, for several values of
 How to Pronounce DKNY? (CORRECTLY)
How to Pronounce DKNY? (CORRECTLY) Better Balance and Stretching Workout DVD - VQ ActionCare - The
Better Balance and Stretching Workout DVD - VQ ActionCare - The UpSpring Post Baby High Waist Postpartum Recovery Underwear - Black - L/XL
UpSpring Post Baby High Waist Postpartum Recovery Underwear - Black - L/XL Womens Skims black Cotton-Rich Dipped Thong
Womens Skims black Cotton-Rich Dipped Thong- The Hilarious World of Funny Panties: A Cheeky Look at the Underwear Revolution
 Lululemon align tank raspberry cream Lululemon align tank, Clothes design, Fashion
Lululemon align tank raspberry cream Lululemon align tank, Clothes design, Fashion
