The entropy change for the conversion of 36 g water to vapour at
4.5 (309) In stock
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1
6. The entropy change for the following reversible process 1 mole H2O(liquid 1atm 100^° c) 1 mole H2(gas,1atm,100^° c)(Δ Hvap=40850j/m) a.+109.52j/k/m b. 109.52j/k/m c.0.00jole/k/mole d.+6.084j/k/m
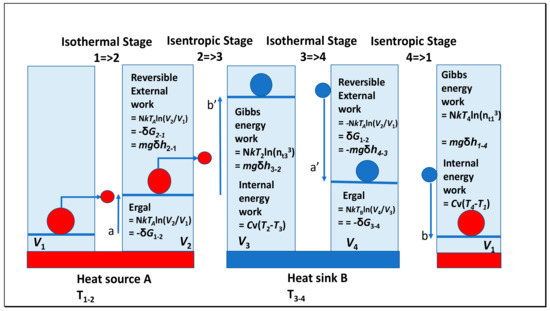
Entropy, Free Full-Text
Why is the change in entropy of the vaporization of water 0 at 373 K? - Quora

The entropy change for the vaporisation of water is 109 J K^(-1) mol^

The entropy change involved in the conversion of 1 mole of liquid water at 373 K to vapour will be:Given: H vap =2.257 kJ / gA. 150 JK 1 mol 1B. 130.6
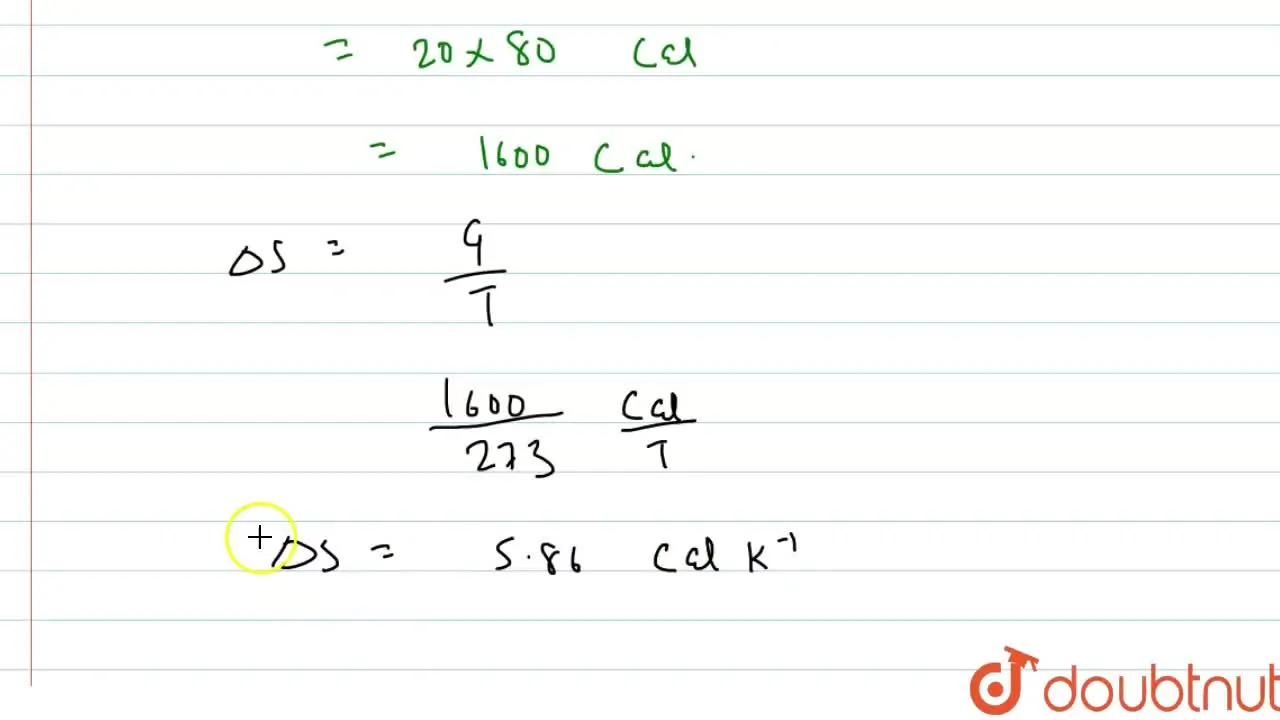
Calculate the entropy change when 20.0 g of ice changes to liquid wate

What is the entropy change (in JK^(-1)mol^(-1)) when one mole of ice i

26. The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 K is (specific heat of water liquid and water vapour are 4.2 kJ

The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 K is : (Specific heat of water liquid and water vapour are 4.2 kJ

The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 Kis: (specific heat of water liquid and water vapour are 4.2 kJ K-kg- and
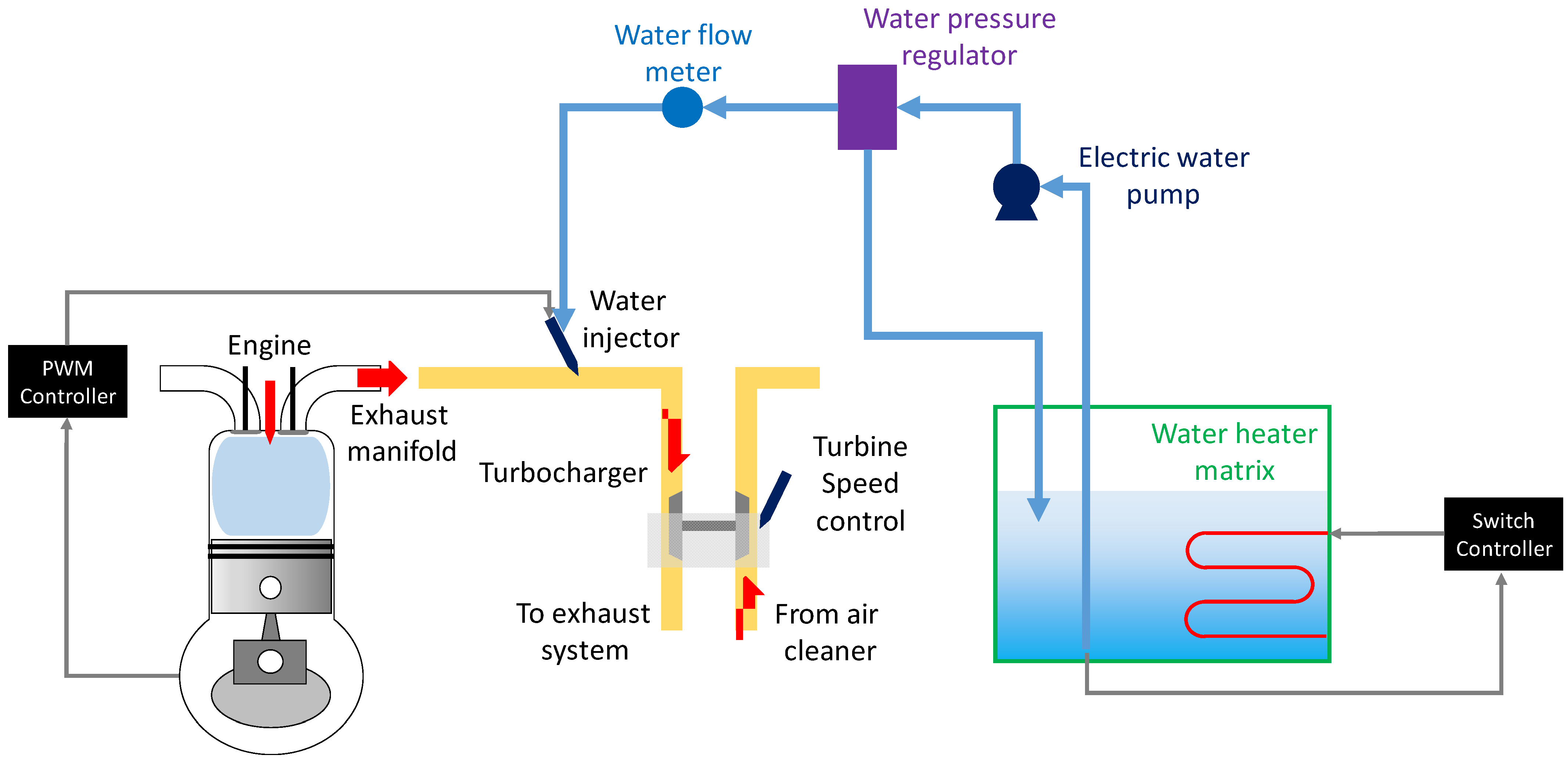
Sustainability, Free Full-Text

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is
Divisora De Massas DV-36 - G.Paniz - Monte Alegre Refrigeração
CARTUCHO L900 36 g IMPACT CALIBRE 12/70 CHUMBO N.° 6 X25 SOLOGNAC
Dia 100 Cura Diarreia Pasta 36 G Real H
 Multinacional Merck está com mais de 30 vagas de estágio abertas
Multinacional Merck está com mais de 30 vagas de estágio abertas Beyond Yoga Spacedye Midi High Waisted Legging Sunny Citrine
Beyond Yoga Spacedye Midi High Waisted Legging Sunny Citrine Contemporary vase - FPF-12 G - FOS CERAMICHE - porcelain / handmade
Contemporary vase - FPF-12 G - FOS CERAMICHE - porcelain / handmade Snowman Mixed Appetizer Plates - Set of 4
Snowman Mixed Appetizer Plates - Set of 4 PLUS/REG Zenana Premium Wide Yoga Wasitband Leggings - Rustic Dove
PLUS/REG Zenana Premium Wide Yoga Wasitband Leggings - Rustic Dove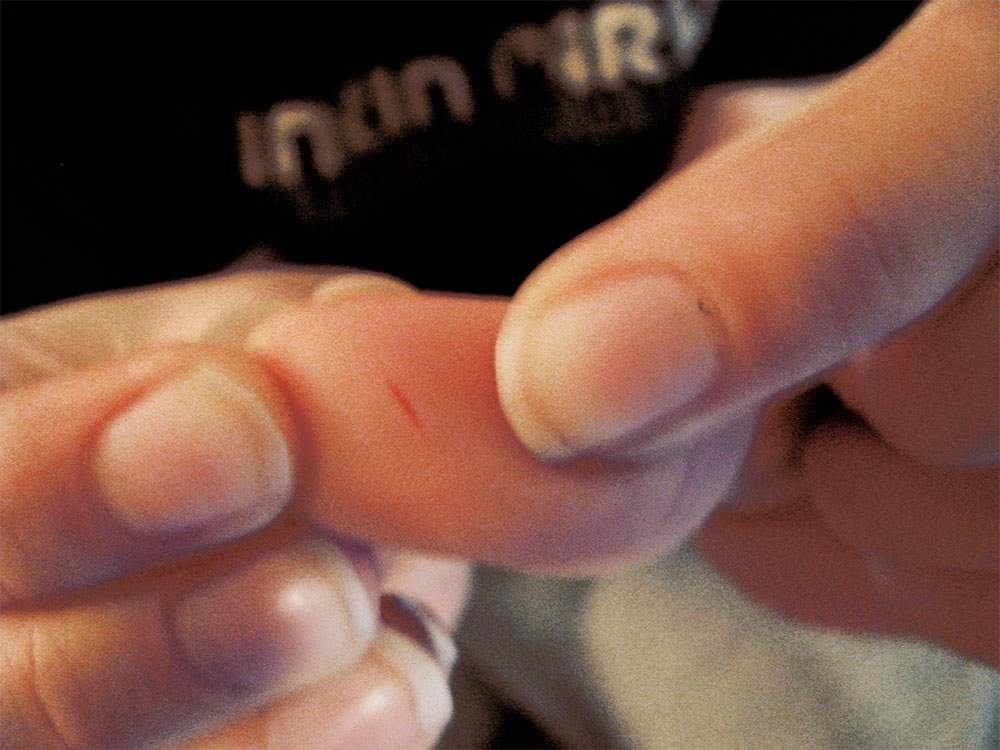 Paper Cuts, Why So Painful? amomentofscience - Indiana Public Media
Paper Cuts, Why So Painful? amomentofscience - Indiana Public Media