The value of compression factor at the critical state of a vander
5 (710) In stock
The value of compression factor at the critical state of a vander waals gas is

The compressibility factor in terms of Pc, Vc and Tc is called Zc. Th

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application
Solved 1, A gas at 250 K and 1 atm has a molar volume 5%
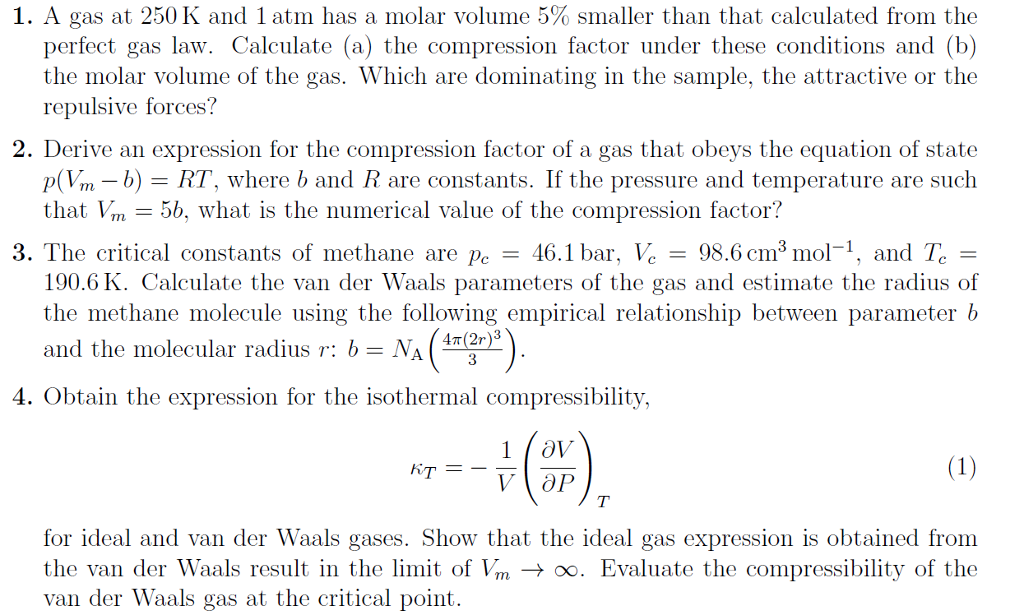
2.7: Real Gases - Chemistry LibreTexts
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
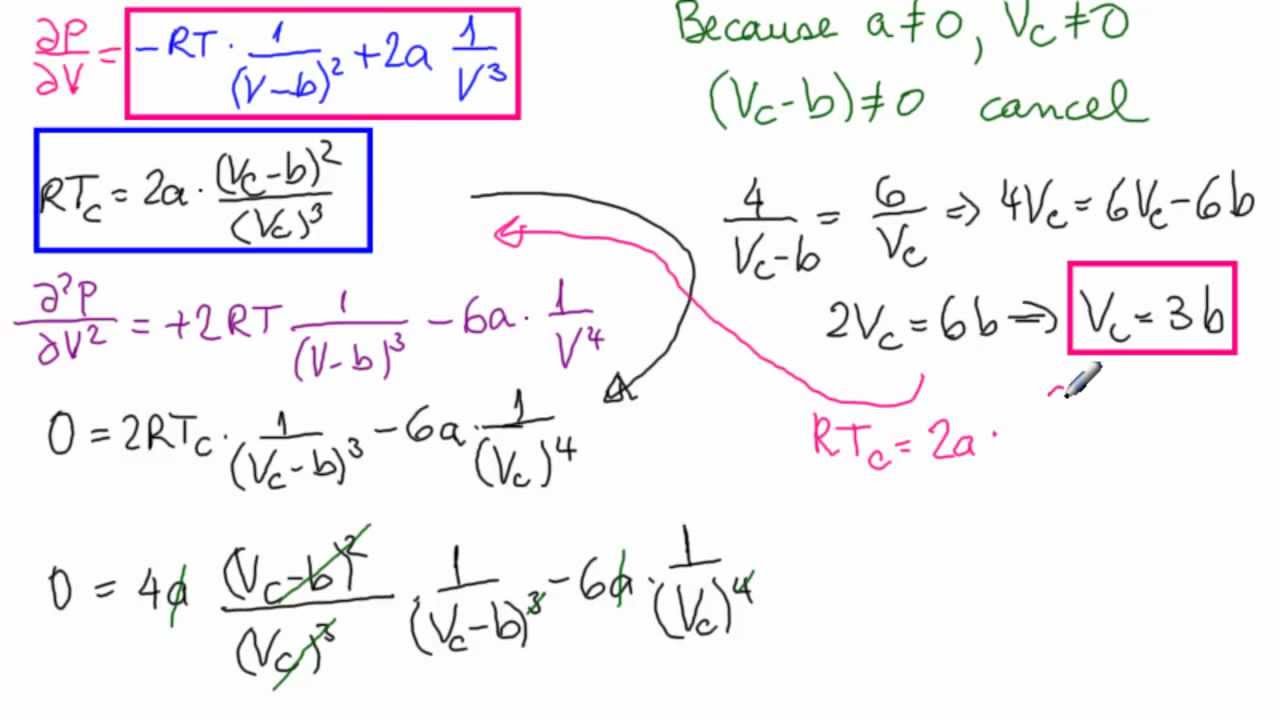
Critical Point with Van der Waal's Equation

1. A Choose the correct option(s) A) At low pressure (nearly 1 atm

Compressibility factor for methane.

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

1. A Choose the correct option(s) A) At low pressure (nearly 1 atm), compressibility factor H, gas is greater than 1 273 K. VB) Compressibility factor a vander Waal's gas its critical
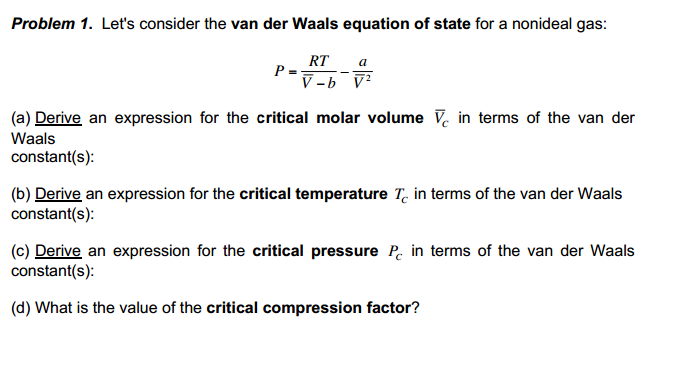
Solved Problem 1. Let's consider the van der Waals equation
How can we calculate critical temperature, volume and pressure in terms of a and b? - Quora
Solved 3) The compressibility factor for a real fluid with
Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes!
Compressibility factors of air using improved virial equation and
Slope of graph of compressibility factor(Z) with pressure(P) for
PDF) New explicit correlation for the compressibility factor of
 Bloomyfit - High Waist Leak Proof Panties,Leak Proof Underwear for Women Incontinence,Plus Size (Color : A, Size : Large)
Bloomyfit - High Waist Leak Proof Panties,Leak Proof Underwear for Women Incontinence,Plus Size (Color : A, Size : Large) Womens Briefs Panties Sexy Sheer Thong Stretchy Underwear Fashion Low Waist Panty Lingerie Plus Size M L XL Grey Black Green From 12,07 €
Womens Briefs Panties Sexy Sheer Thong Stretchy Underwear Fashion Low Waist Panty Lingerie Plus Size M L XL Grey Black Green From 12,07 € Faux-Fur Lined Bomber Jacket
Faux-Fur Lined Bomber Jacket Brilliant Basics Women's T-Shirt Bra 2 Pack - Nude & White - Size
Brilliant Basics Women's T-Shirt Bra 2 Pack - Nude & White - Size Bodysuits for Support and Seamless Tucked-in
Bodysuits for Support and Seamless Tucked-in Being Runner Women Non-See Through Sportswear Sweatpants | Wine Yoga Tights | Black Gym Legging | Plus Size Comfort Jeggings With Pocket (Black With
Being Runner Women Non-See Through Sportswear Sweatpants | Wine Yoga Tights | Black Gym Legging | Plus Size Comfort Jeggings With Pocket (Black With