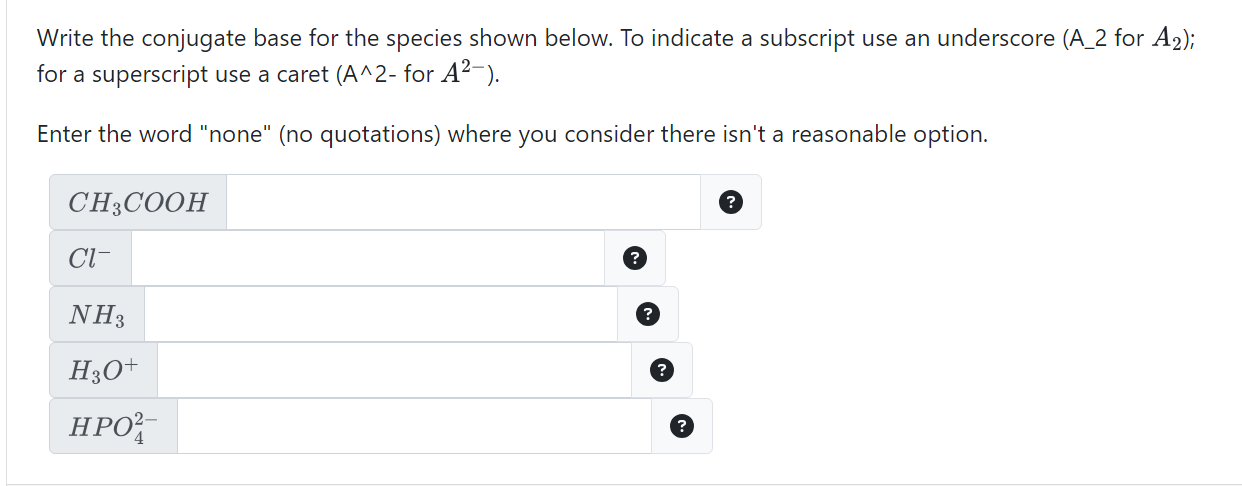
Solved Write the conjugate base for the species shown below
4.7 (555) In stock
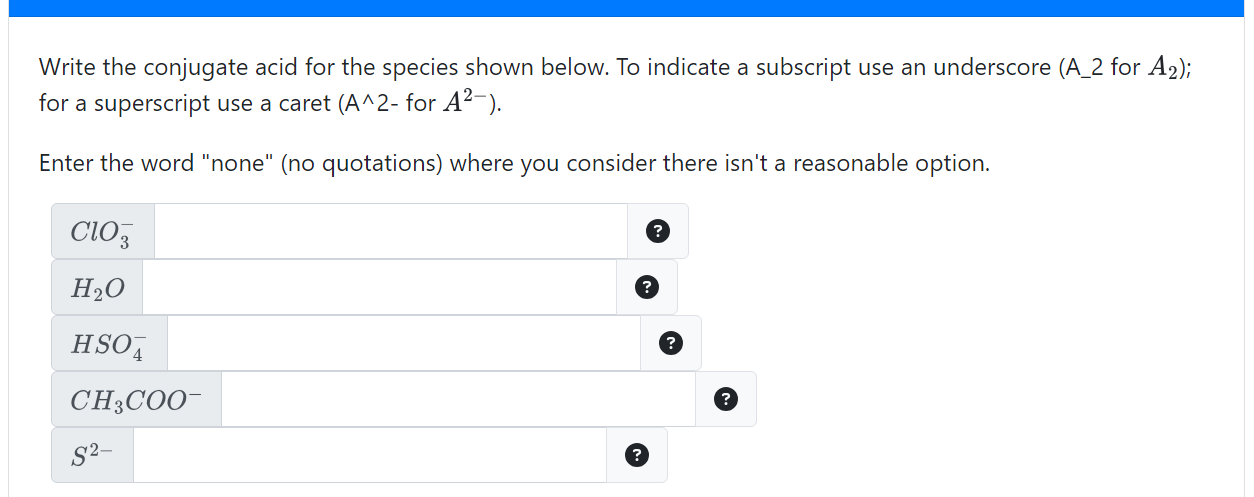
Solved Write the conjugate acid for the species shown below.

Caffeine (C8H10N4O2) is a weak base with a pKb of 10.4. Calculate
/chapter3/pages33and34/page33and34_files/aqh3o.png)
Chapter 3

The following aqueous species constitute two conjugate acid

For the chemical equations shown below, label each reactant as either acid or base, and each product as either conjugate acid or conjugate base according to the Bronsted-Lowry definition. [{Image src

14.9b How to identify the conjugate acid-base pairs in CN− + H2O → HCN + OH−
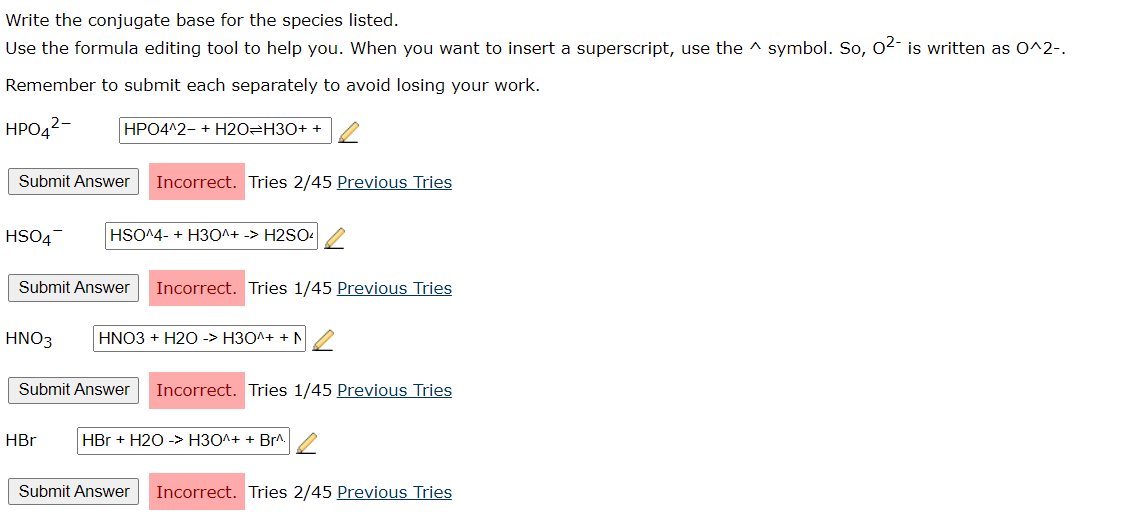
Solved Write the conjugate base for the species listed. Use
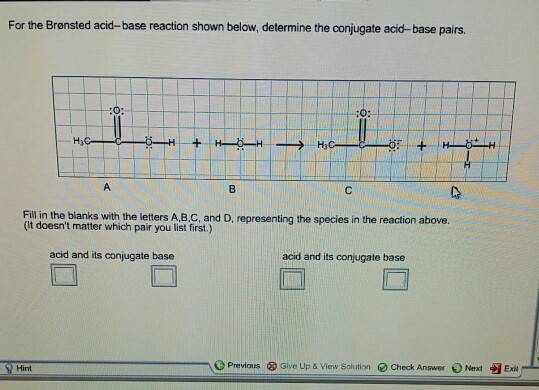
Solved For the Bronsted acid-base reaction shown below
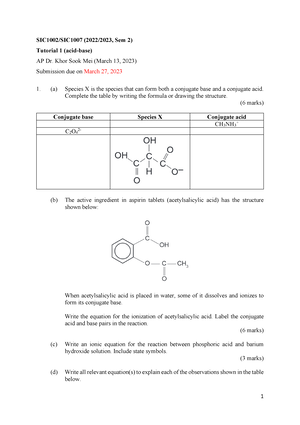
Tutorial 1 (SIC1002 SIC1007) (20222023 Sem 2) - 1 SIC1002/SIC1007 (2022/2023, Sem 2) Tutorial 1 - Studocu
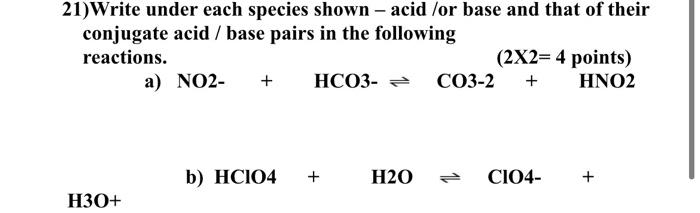
Solved 21)Write under each species shown - acid /or base and
How to find the conjugate base of acetic acid - Quora

4.8 Introduction To Acid-Base Reactions Student, PDF, Acid
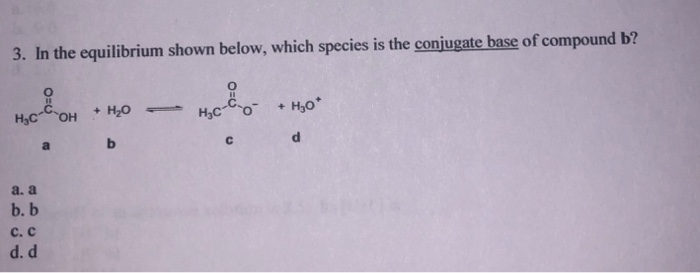
Solved 3. In the equilibrium shown below, which species is

Write the reaction of glycine acting as a base. Show the structure of glycine under physiological conditions. Include charges. Omit lone pairs and explicit hydrogens.
Underscore-Beloved Family Portrait (Tubbo, Ranboo, and Michael) | Pillow
Underscore Island Pendant by Metropolitan Lighting, N6959-1-267B
Cat by vicnick-underscore on DeviantArt
Metropolitan N6952 3 Light Bowl Shaped Pendant from the Underscore
 Cotton Clothing Art Women Co Ord Top Pant Set, Hand Wash, 140 Gsm at Rs 499/set in Rajkot
Cotton Clothing Art Women Co Ord Top Pant Set, Hand Wash, 140 Gsm at Rs 499/set in Rajkot Evan Slim Fit Nubuck Beige Blazer – MCR TAILOR
Evan Slim Fit Nubuck Beige Blazer – MCR TAILOR Victoria Beckham shines in a Studio 54-inspired slip at Brooklyn Beckham's wedding
Victoria Beckham shines in a Studio 54-inspired slip at Brooklyn Beckham's wedding 10 Types of women Trousers Are a Must-have for All Women
10 Types of women Trousers Are a Must-have for All Women The Country Girl (1954 film) - Wikipedia
The Country Girl (1954 film) - Wikipedia Losha Wire-Free Nursing Bra - PlazzaPK Lifestyle
Losha Wire-Free Nursing Bra - PlazzaPK Lifestyle