SOLVED: Derive an expression for the compression factor of a gas
4.9 (784) In stock

VIDEO ANSWER: And this question we're going to be dealing with the equation state equation of state where P multiplied by V minus n B. Is equality and are a team. So we're dealing with a scenario where VM is equal to 10 B. So what would have right
Derive an expression for the compression factor of a gas that obeys the equation of state p(V - nb) = nRT, where b and R are constants. If the pressure and temperature are such that Vm = 10b, what is the numerical value of the compression factor?
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
PDF) Step by Step Derivation of the Optimum Multistage Compression

Deviation from ideal gas behaviour
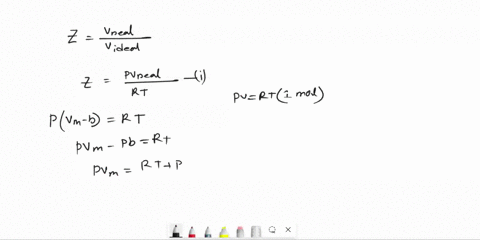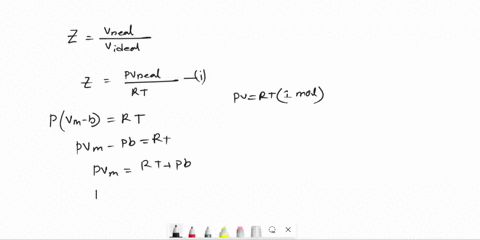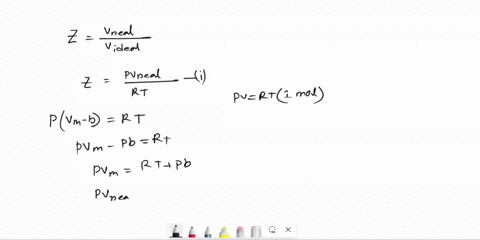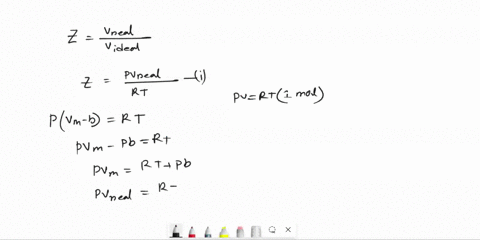
SOLVED: Derive an expression for the compression factor of a gas that obeys the equation of state p(V-nb)=nRT, where b and R are constants. If the pressure and temperature are such that

How to Calculate Compression Ratio: 9 Steps (with Pictures)

Isothermal compressibility function of pressure and temperature
Isentropic exponent κ for hypothetical natural gas mixture with

Van der Waals equation - Wikipedia

Van der Waals Equation, Definition & Examples - Lesson
Derivation of the barometric formula (adiabatic atmosphere) - tec

SOLVED: Derive an expression for the compression factor of a gas
Equations of Compressible and Incompressible Flow in Fluid
.jpg)
Answered: problem 19.5 545 J of work must be…

Gas Flow in Control Valves

Topic 2 kft 131
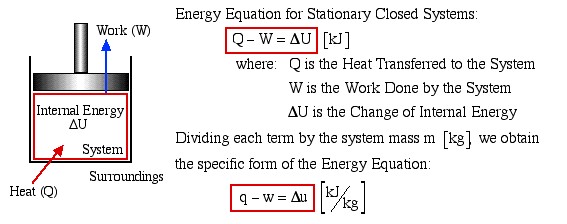
Chapter 2: The First Law of Thermodynamics for Closed Systems
Solved The Berthelot equation of state is given by
 DR. Brandt Needles No More Neck Sculpting Cream (with Gua Sha Tool
DR. Brandt Needles No More Neck Sculpting Cream (with Gua Sha Tool Teen Girl Bra Pink Kawaii Lace Floral Bras Push Up Bralette Wireless Underwear Seamless Brassiere Student
Teen Girl Bra Pink Kawaii Lace Floral Bras Push Up Bralette Wireless Underwear Seamless Brassiere Student French Laundry Women's Leggings. Plus Sizes Available (2-Pack
French Laundry Women's Leggings. Plus Sizes Available (2-Pack The Little Mermaid Costume Designer on Why Halle Bailey Doesn't Wear Seashell Bikini in $414
The Little Mermaid Costume Designer on Why Halle Bailey Doesn't Wear Seashell Bikini in $414 All sports Stock Photos, Royalty Free All sports Images
All sports Stock Photos, Royalty Free All sports Images Street style - Jordan Looks estilosos, Looks vintage femininos, Looks
Street style - Jordan Looks estilosos, Looks vintage femininos, Looks