Compressibility factor Z = PV / nRT is plotted against pressure as
4.5 (672) In stock

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

As the pressure approaching zero i.e., very low pressure, the curves plotted between compressibility factor Z and P n mole of gases have the following characteristics.I. The intercept on the y-axis leads

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following

shows plots of the compressibility factor, Z = P V /RT , of methanol at
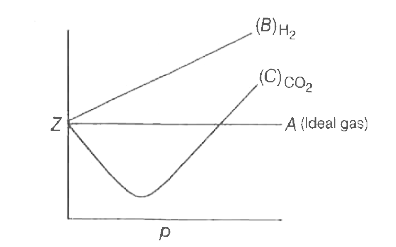
Telugu] The variation of compressibility factor (Z) with pressure (p

Gas Compressibility - an overview

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
1.5 Real Gases and the Virial Equation - Mail

Compressibility factor - Wikipedia

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees
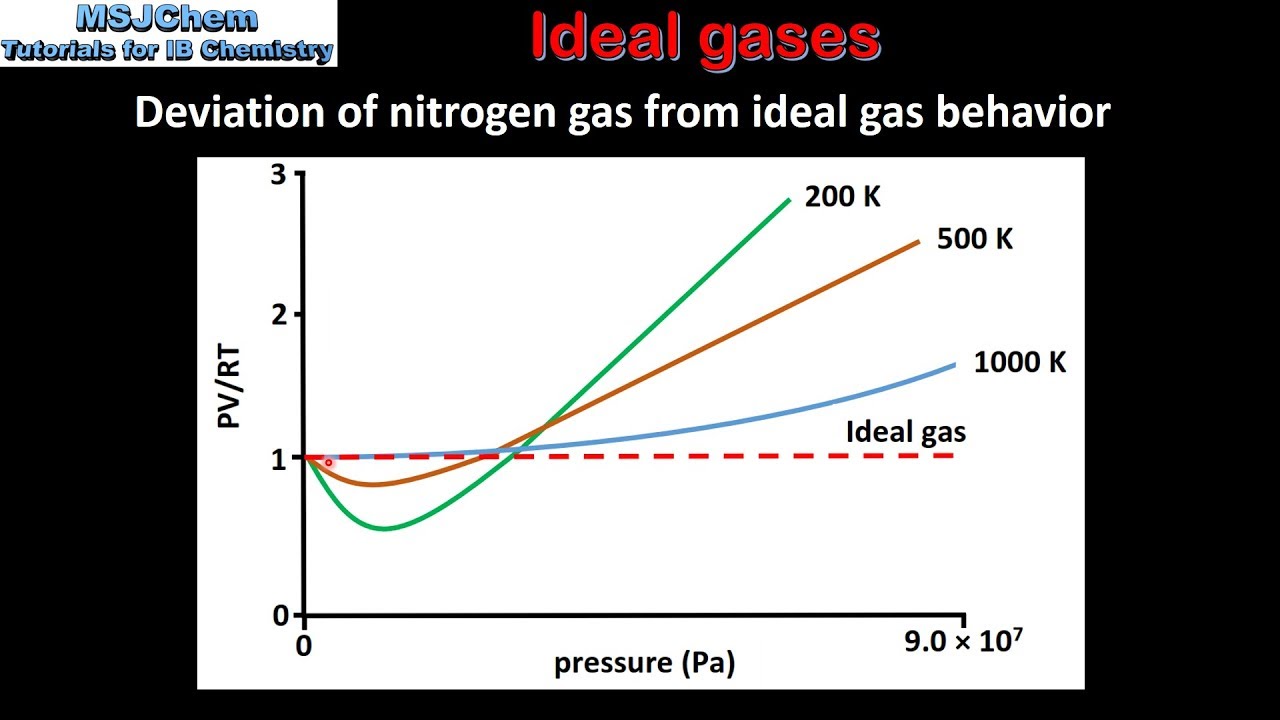
1.3 Deviation from ideal gas behaviour
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
Essential Pharma Documents: 1205: Properties of Gases
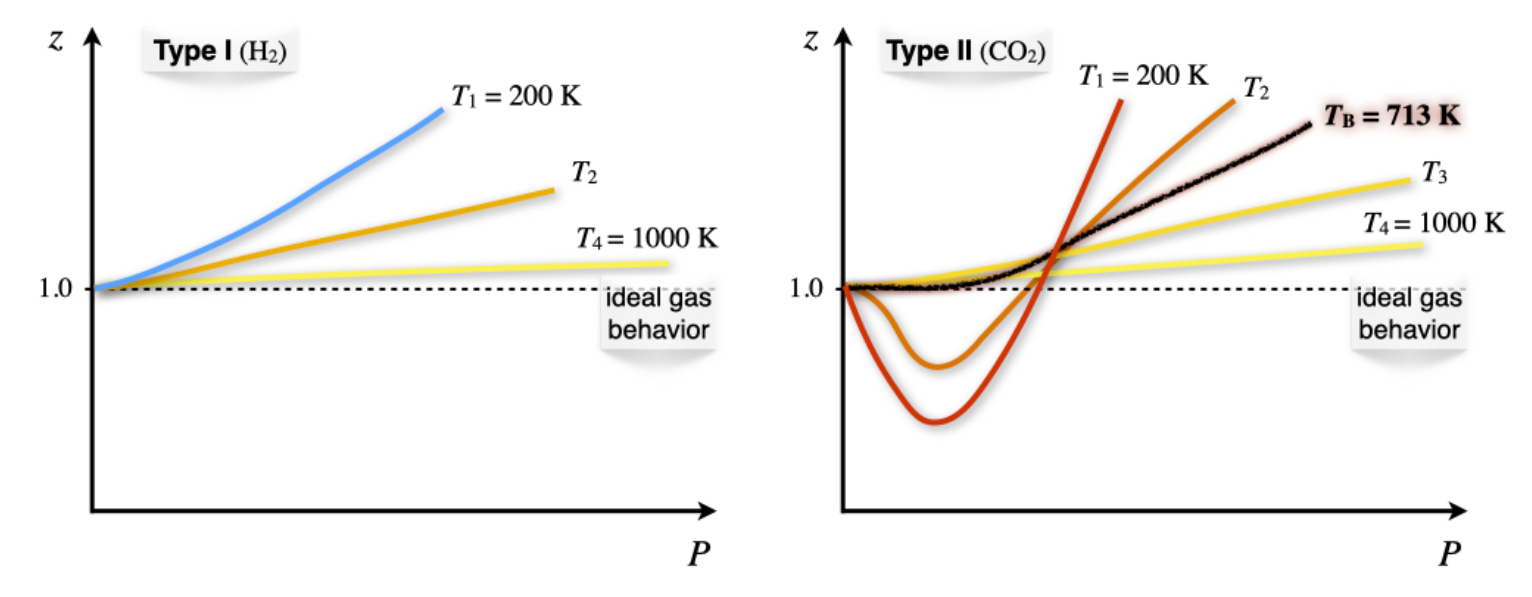
11.3: Critical Phenomena - Chemistry LibreTexts
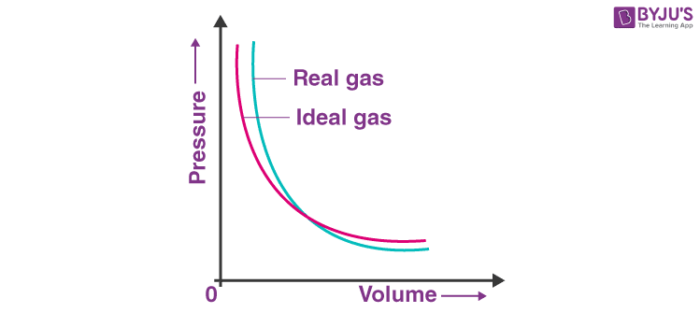
Deviation Of Real Gas From Ideal Gas Behavior
Determine Compressibility of Gases
3.2 Real gas and compressibility factor – Introduction to
Real gasses For an ideal gas, the compressibility factor Z = PV
Super-critical Fluid Compressibility Factor Z , for Intermediate
 Jordan Brand Jordan Brand x Travis Scott Lace Pants Dark Smoke
Jordan Brand Jordan Brand x Travis Scott Lace Pants Dark Smoke USA Pro, Gloss Sports Bra, Black Animal
USA Pro, Gloss Sports Bra, Black Animal- Lululemon Hotty Hot Low-rise Lined Shorts 4 In Sonic Pink
 NWOT - Lululemon Align Jogger Crop *23 Dark Olive | SIZE: 2, 4, 6
NWOT - Lululemon Align Jogger Crop *23 Dark Olive | SIZE: 2, 4, 6 XELORNA Bootcut Yoga Dress Pants for Women Stretchy Work Pants Casual Slacks Trousers for Office Business with 6 Pockets - ShopStyle
XELORNA Bootcut Yoga Dress Pants for Women Stretchy Work Pants Casual Slacks Trousers for Office Business with 6 Pockets - ShopStyle Stainless Steel Brazil Map Pendant Necklace Brasil Map
Stainless Steel Brazil Map Pendant Necklace Brasil Map
