The compression factor (compressibility factor) for 1 mol of a van der
4.9 (751) In stock

For 1 mol of a gas, the van der Waals equation is (P+(a)/(V(m)^(2)))(V(m)-b)=RT Ignoring b, we get (given volume of gas molecule is negligible) (P+(a)/(V(m)^(2)))V(m)=RT ltbgt or pV(m)+(a)/(V(m))=RT or (pV(m))/(RT)+(a)/(V(m)RT)=1 or Z=(pV(m))/(RT)=1-(a)/(V(m)RT) (i) It is given that Z=(pV(m))/(RT)=0.5implies V(m)=(0.5RT)/(P) With this, equation (i) becomes 0.5=1-(a)/((0.5RT//p)RT) or a=(0.5)((0.5RT)/(p))RT=0.25(R^(2)T^(2))/(p) Substiuting the given values, we get a=(0.25)[((0.082L atm K^(-1)mol^(-1))^(2)(273 K)^(2))/((100 atm))] =1.2528 L^(2) atm mol^(-2)
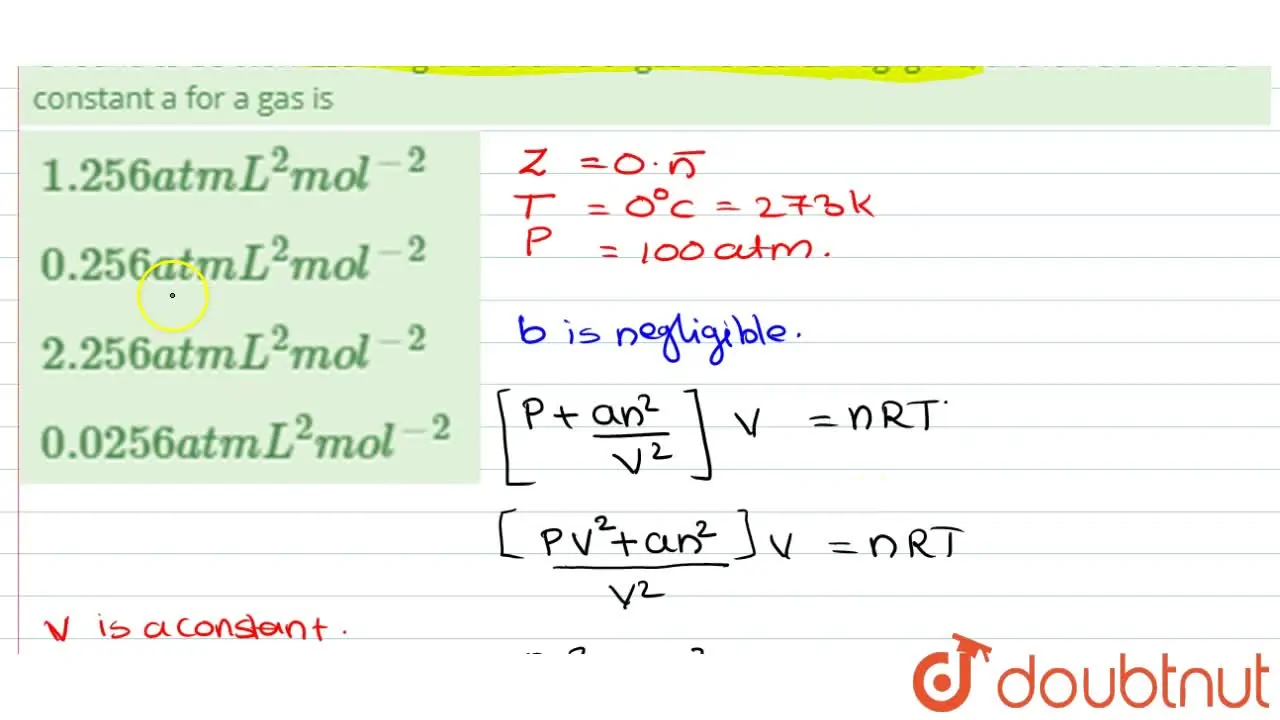
Malayalam] The compressibility factor for definite amount of van der

The density of the vapour of a substance at 1 atm pressure and 500 K i
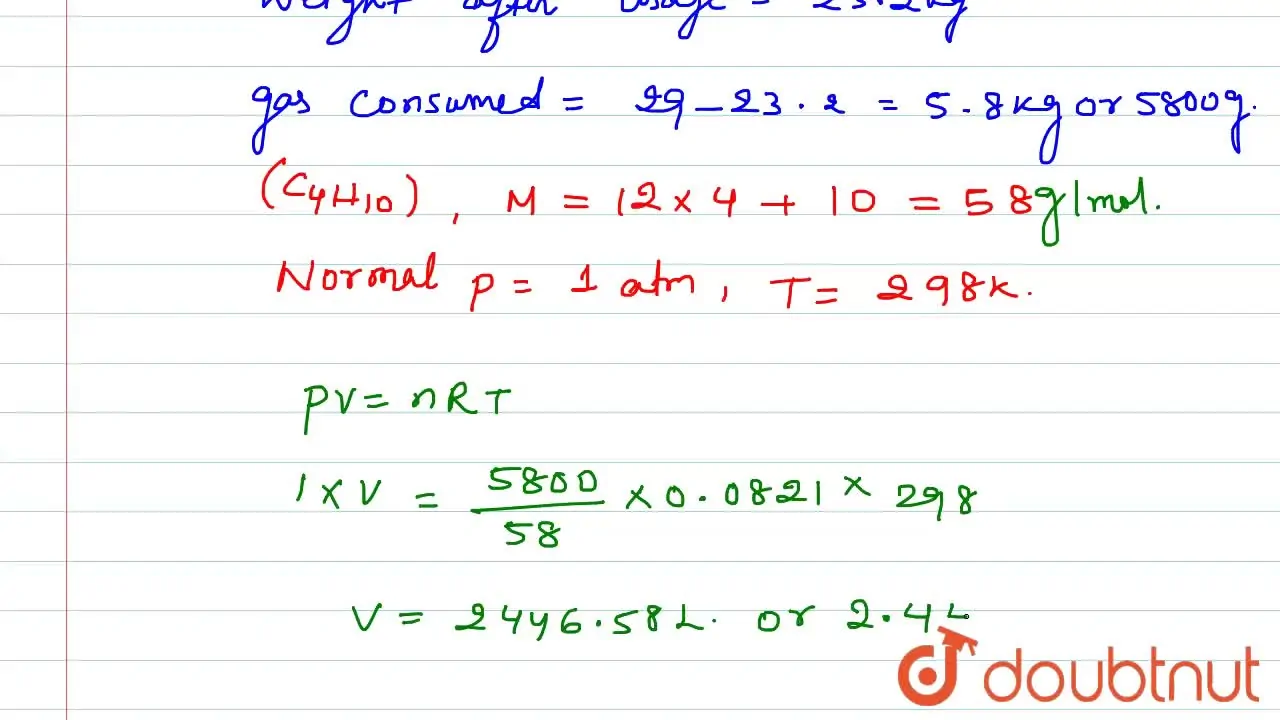
An LPG cylinder weighs 14.8 kg when empty. When full it weighs 29.0 kg
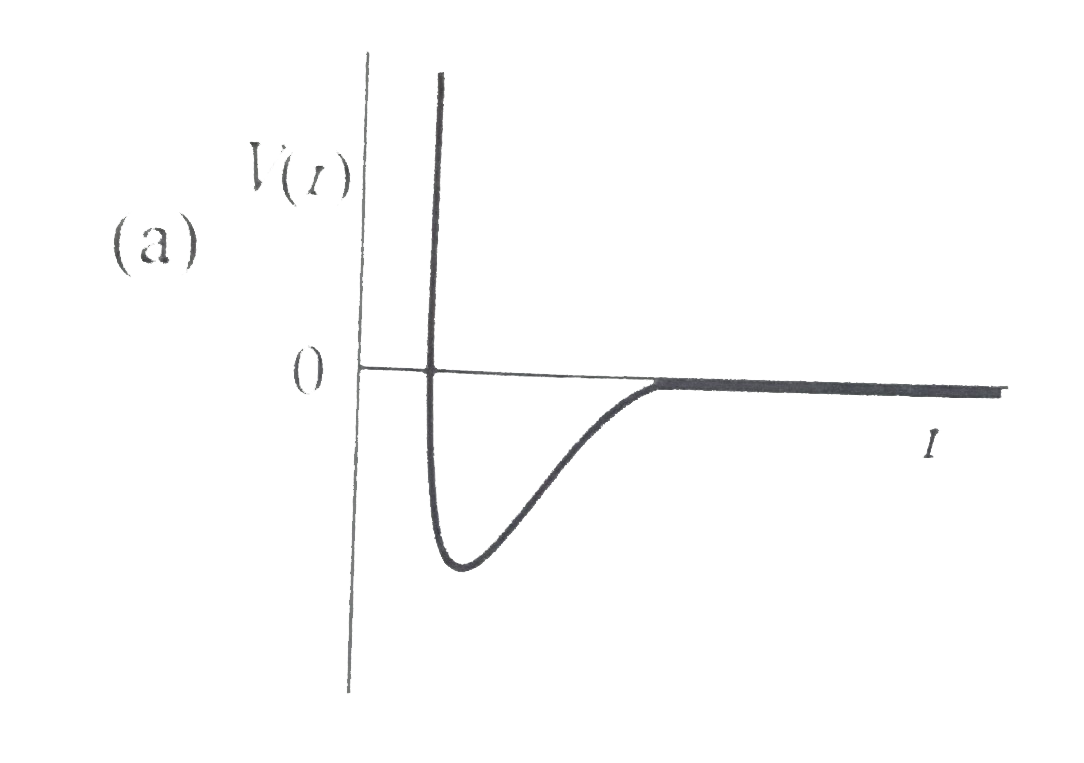
One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh
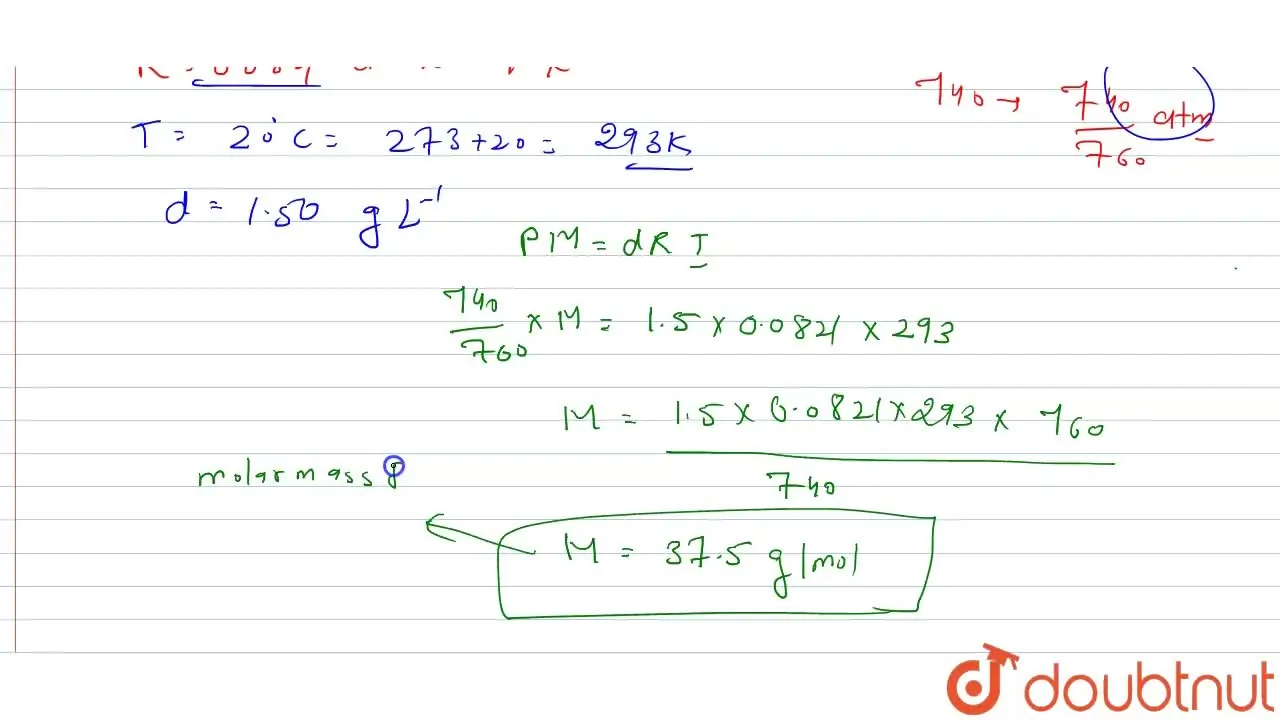
A mixture of CO and CO(2) is found to have a density of 1.50 g L^(-1
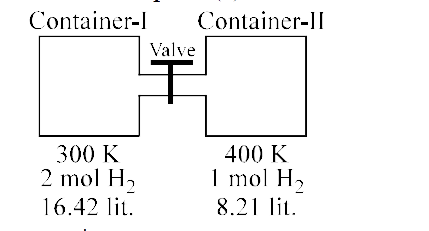
Moles in each compartment are same after opening the valve.
An LPG cylinder weighs 14.8 kg when empty. When full it weighs 29.0 kg
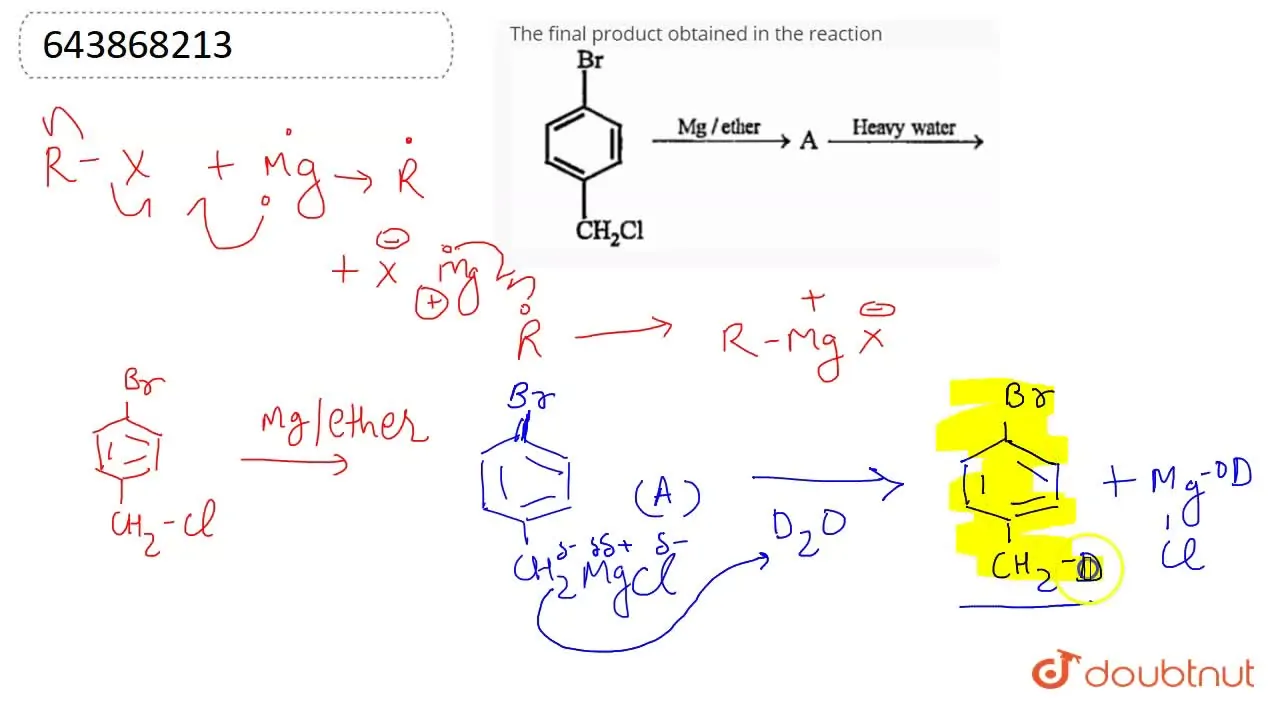
The final product obtained in the reaction

At 30^(@)C and 720 mm and Hg, the density of a gas is 1.5 g// l t. Cal
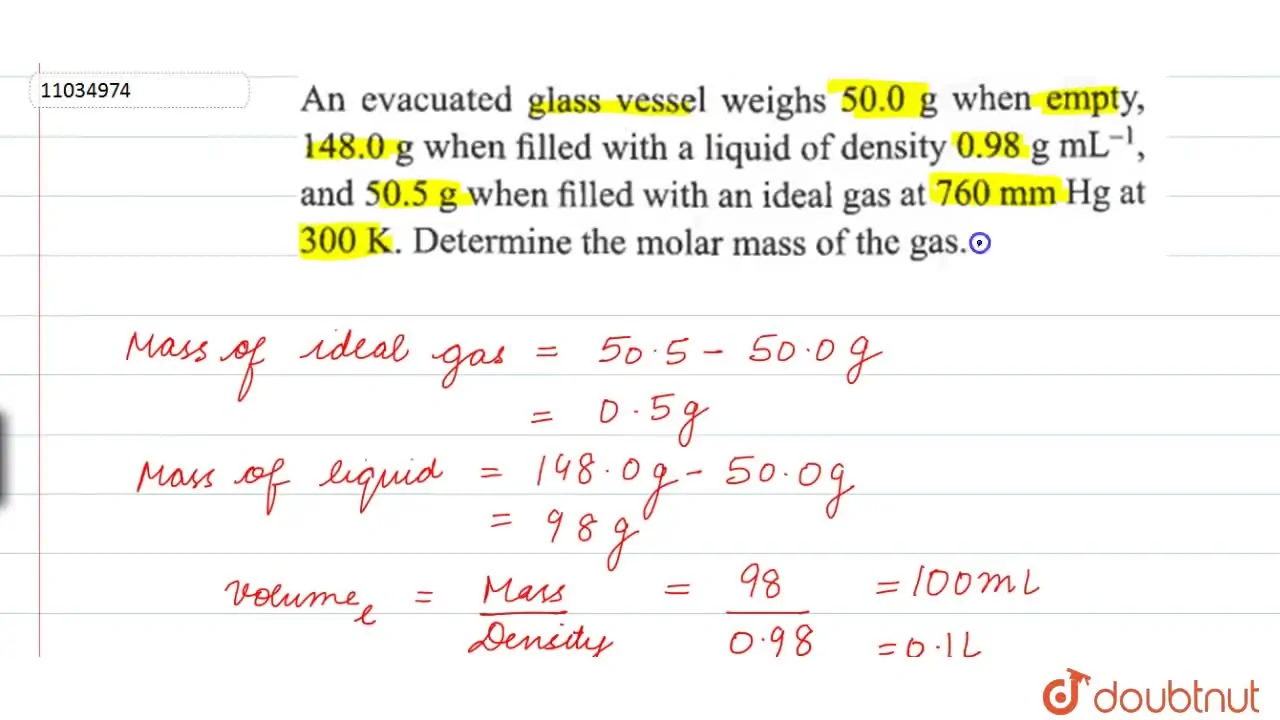
An evacuated glass vessel weighs 50.0 g when empty, 148.0 g when fille
Solved 2. By definition, the compression factor of an ideal
Solved As a first approximation, the compression factor, Z
Solved 9 Compression factor Z Use the van-der-Waals equation
Solved 1. Consider the expression of the following
Find the isothermal compressibility `x` of a Van der Walls gas as a function of volume
 No Boundaries Seamless Camisoles for Women
No Boundaries Seamless Camisoles for Women Washable Women's Incontinence Underwear - Odor-lock Fabric Technology
Washable Women's Incontinence Underwear - Odor-lock Fabric Technology Kendall Jenner and Devin Booker Spark Marriage, Pregnancy Rumors - PAPER Magazine
Kendall Jenner and Devin Booker Spark Marriage, Pregnancy Rumors - PAPER Magazine Under Armour Women's Pure Stretch Thong 3 Pack –
Under Armour Women's Pure Stretch Thong 3 Pack – Body Feminino Sem Bojo Alça Cruzada Renda Verde
Body Feminino Sem Bojo Alça Cruzada Renda Verde Dickies Men's Loose Fit Cargo Work Pant, Black, 30x30
Dickies Men's Loose Fit Cargo Work Pant, Black, 30x30