Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart from ideal. - ppt download
4.9 (498) In stock
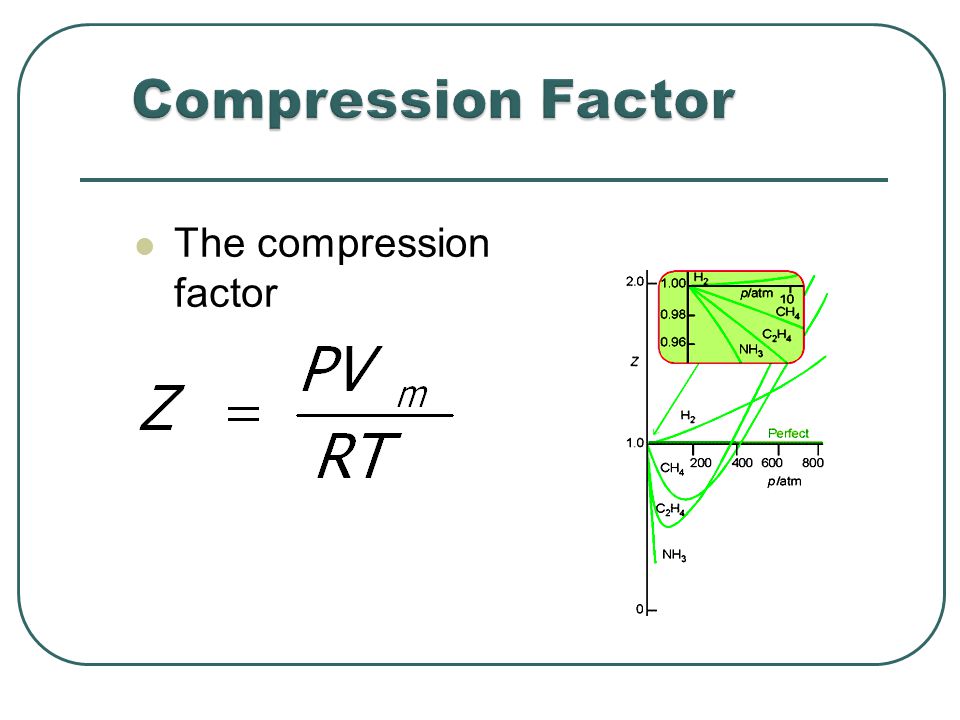
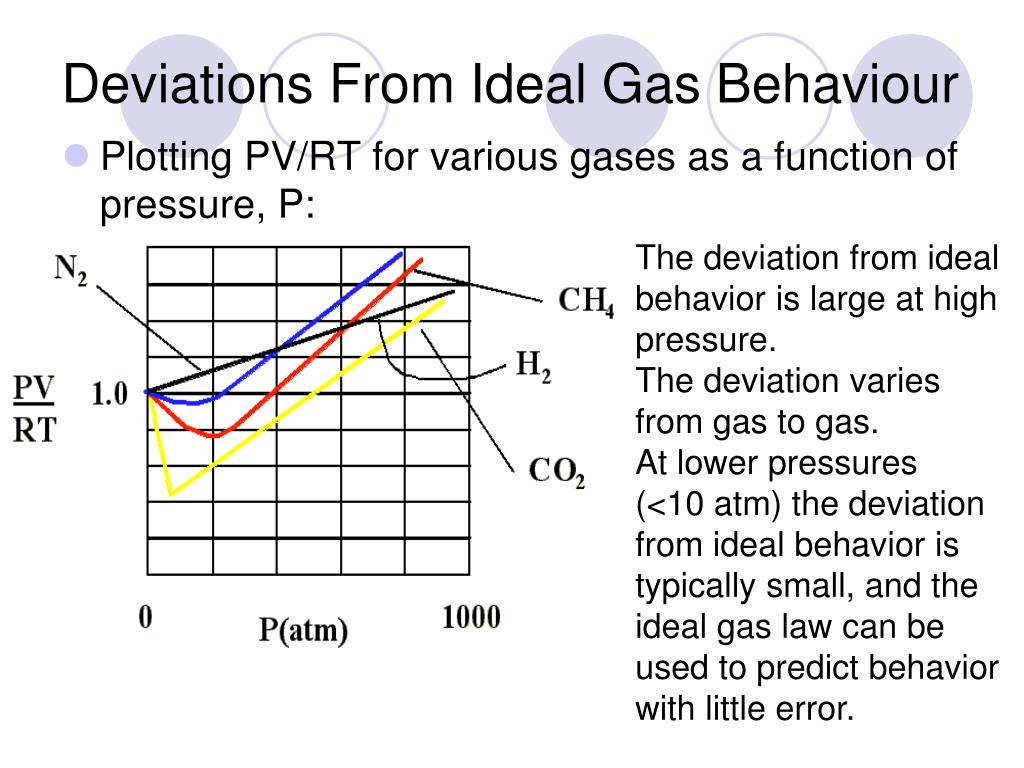
Most real gases depart from ideal behaviour at deviation from low temperature high pressure.
High positive potential energy (little separation) Repulsive interactions Intermediate separations attractive interactions dominate Large separations (on the right) the potential energy is zero and there is no interaction between the molecules..
Real gas molecules do attract one another (P id = P obs + constant) Real gas molecules are not point masses (V id = V obs - const.)
V id = V obs - nb b is a constant for different gases P id = P obs + a (n / V) 2 a is also different for different gases Ideal gas Law P id V id = nRT
Critical temperature (T c ) - the temperature above which a gas cannot be liquefied Critical pressure (P c ) – the minimum pressure that needs to be applied at T c to bring about liquefaction
For a perfect gas, the slope is zero Boyle temperature the slope is zero and the gas behaves perfectly over a wider range of conditions than at other temperatures.
Boyle temperature - for a van der Waal s gas, the Boyle temperature (T B ) is written
The reduced state variables are defined
Re-write the Van der Waals in terms of reduced variables
The chemical potential of a real gas is written in terms of its fugacity
In gaseous systems, we relate the fugacity (or activity) to the ideal pressure of the gas via.
Define the fugacity coefficient = f / P For a real gas.
Comparing the chemical potential of the real gas to the chemical potential of an ideal gas at the same pressure
The fugacity coefficients are obtained from the compression factors (Z) as shown below

Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart from ideal. - ppt download

Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart from ideal. - ppt download

PPT - Real Gases: Deviations from Ideal Behavior PowerPoint Presentation - ID:2093358

Kinetic Theory Class 11 Notes CBSE Physics Chapter 13 (PDF)

Deviation of real gas from ideal behaviour

14.11: Real and Ideal Gases - Chemistry LibreTexts
PPT - REAL VS IDEAL GASES PowerPoint Presentation, free download - ID:1430890
Real vs. Ideal Gases — Comparison & Importance - Expii

Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart from ideal. - ppt download
7.2 Ideal gas laws, Ideal gases

Analysis of Real Gas Behavior Using the van der Waals Equation of State: A Computational Study of Ethyl Acetate, PDF, Gases

Ideal & real gases
Derive an expression for the compression factor of a gas tha
Find the isothermal compressibility `x` of a Van der Walls gas as a function of volume
the compression factor one mole of a vander waals gas 0 C and 100
 No Boundaries NOBO Buffalo Plaid Juniors Red Black Leggings NWT szS/M/L/XL/2X/3X
No Boundaries NOBO Buffalo Plaid Juniors Red Black Leggings NWT szS/M/L/XL/2X/3X Joyspun Women's & Women's Plus Size Underwire T-Shirt Bra, Sizes 38DD to 46DDD
Joyspun Women's & Women's Plus Size Underwire T-Shirt Bra, Sizes 38DD to 46DDD- adidas by Stella McCartney storms into the new season with the launch of its Fall/Winter 2018 collection
 Deep Red Silk Camisole With Black Lace, Silk Satin Camisole
Deep Red Silk Camisole With Black Lace, Silk Satin Camisole Mint AQUA Activewear – LORETA
Mint AQUA Activewear – LORETA Womens Bras Clearance Women Solid Underwired Sexy Lace Lingerie Everyday Bras Tube Top Sling Inner Wrap Chest Vest with Chest Pad French Underwear Daily Bra Black : : Fashion
Womens Bras Clearance Women Solid Underwired Sexy Lace Lingerie Everyday Bras Tube Top Sling Inner Wrap Chest Vest with Chest Pad French Underwear Daily Bra Black : : Fashion