the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians
4.9 (604) In stock

the equation of state of a gas is p(v-nb)=rt where b and r are constants. if the pressure and temperature are such that vm=10b what is the value of compressibi

In the gas equation, (P +dfrac{a}{V^2}) (V - b) = RT , where P is pressure, V is volume, R is gas constant and T is temperature. Calculate the dimensional formula of
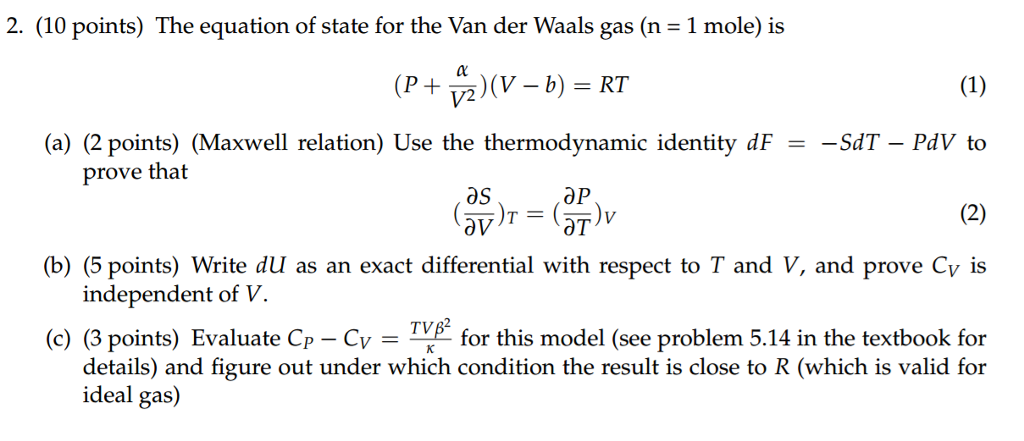
Solved The equation of state for the Van der Waals gas (n =
Deviation From Ideal Gas Behavior - Study Material for IIT JEE

SOLVED: The equation of state of a certain gas is given by: P + b = RT. What is the entropy change for an isothermal process? Hint: you can use the first

aieee04

Physical Chemistry- Revision Notes on Gaseous State for IIT JEE & Other Engineering Exams

Gaseous State Notes, PDF, Gases

372 The equation of state of a gas is given by P V C = (RT + b), where a, b, c and R are constants. The isotherms can be represented by
How is the Combined Gas Law derived? - Quora

aieee04

The equation of state of a real gas is given by p+ (V - b)=RT, where p. V and T are pressure, volume and temperature respectively and R is the universal gas

Example 15 The equation of a state of a real gas is given by P +- (V - b) = RT, where T is absolute temperature, P is pressure, V is volume

The equation of state for a gas is P(V−nb)= nRT, where b&R are constant. ..
Solved Show that the compressibility factor of van der Waals
Compressibility Factor Calculator - File Exchange - MATLAB Central
Compressibility factor (gases) - Citizendium
Gas Compressibility Factor Spreadsheet Calculator
Compressibility factors of air using improved virial equation and




