Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
4.5 (280) In stock


Deviation of real gas from ideal behaviour

Properties of Gas Manik

Assignment gaseous state_jh_sir-2621

Gaseous State JEE, PDF, Gases

Properties of Gas Manik
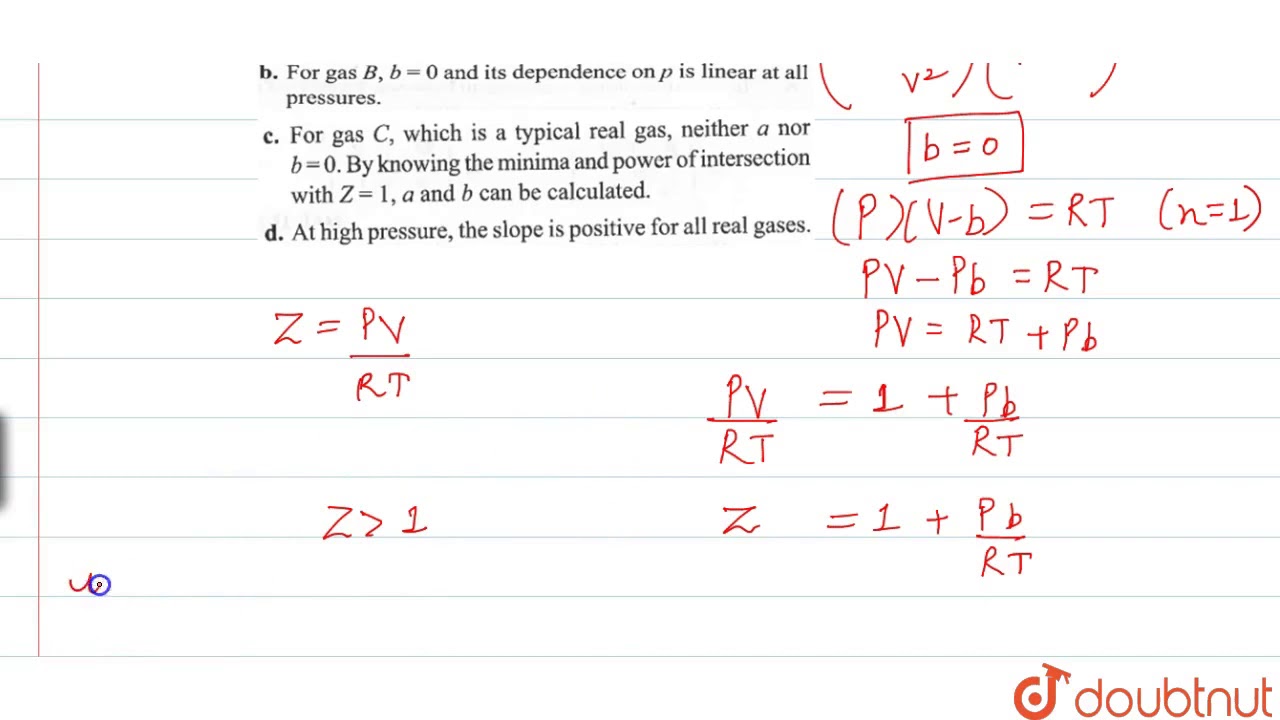
The given graph represents the variations of compressibility

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Ideal Gases & Real Gases, PDF, Gases

The compressibility factor of a gas is defined as Z=P V / R T. The

Gas compressibility factor Z: Ideal gas vs Real gas

Properties of Gas Manik
Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor
PPT - GASES PowerPoint Presentation, free download - ID:2088317
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT
) Buy REBANTA Mens Casual Shirt Double Pocket (Red, Pure Cotton,4XL
Buy REBANTA Mens Casual Shirt Double Pocket (Red, Pure Cotton,4XL Buy D'chica Strong Unicorn Print Period Panties for Teenagers-Maroon online
Buy D'chica Strong Unicorn Print Period Panties for Teenagers-Maroon online Victoria's Secret Panties, Knickers, Underwear Various Styles
Victoria's Secret Panties, Knickers, Underwear Various Styles Lily James reveals the Mamma Mia dances each took a week to film
Lily James reveals the Mamma Mia dances each took a week to film Mélange – Charcoal
Mélange – Charcoal Sexy Lace Crotchless Briefs Transparent Back Open Crotch Panties Floral Women Underwear Sexy Lingerie Mujeres Ropa Interior BY DHL From Harrypotter_jewelry, $1.22
Sexy Lace Crotchless Briefs Transparent Back Open Crotch Panties Floral Women Underwear Sexy Lingerie Mujeres Ropa Interior BY DHL From Harrypotter_jewelry, $1.22