What is the change in internal energy (in J) of a system that absorbs 0.464 kJ of heat from its surroundings and has 0.630 kcal of work done on it?
4.7 (197) In stock

I found an increase of 3100J Have a look

What is the change in internal energy (in J) of a system that
PDF) Resilient and Sensitive Key Points of the Photosynthetic
1. Calculate the internal energy change for each of the following

15.4 What is the change in internal energy of a system which
A system absorbs 180 J of heat and does 160 J of work. What is the

The elastic properties, elastic models and elastic perspectives of

Industrial Color Testing (2nd Edition), PDF, Pigment
Calculate the change in internal energy of a system if the energy

Handbook on Energy Conscious Buildings by Supplementary
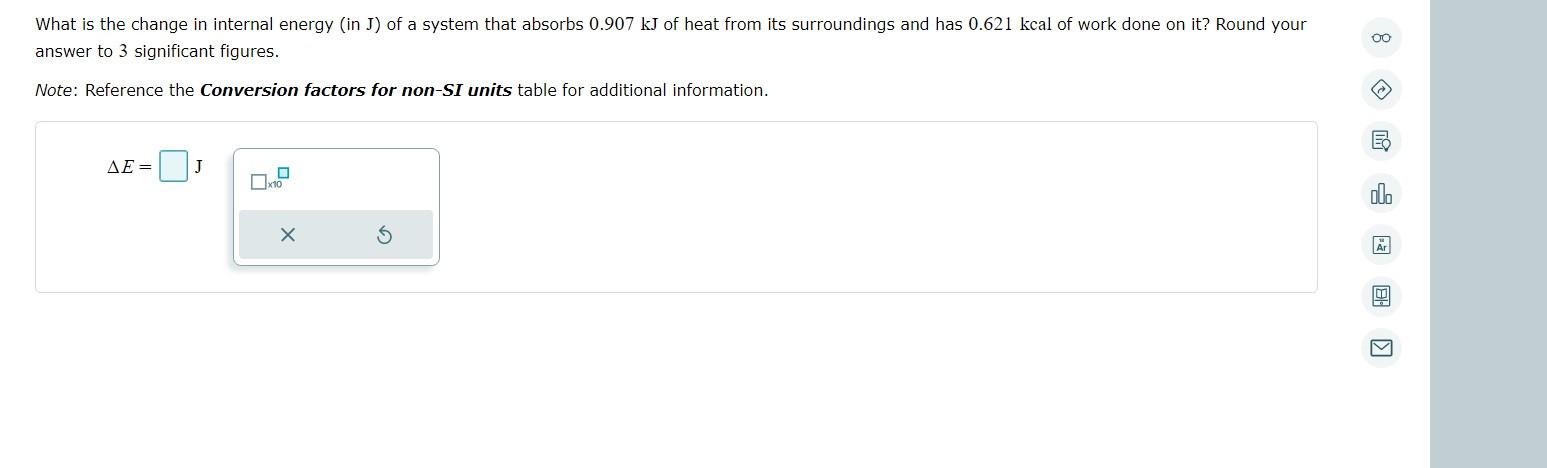
Solved What is the change in internal energy (in J) of a

Solved Be sure to answer all parts. What is the change in

chemia - Studia
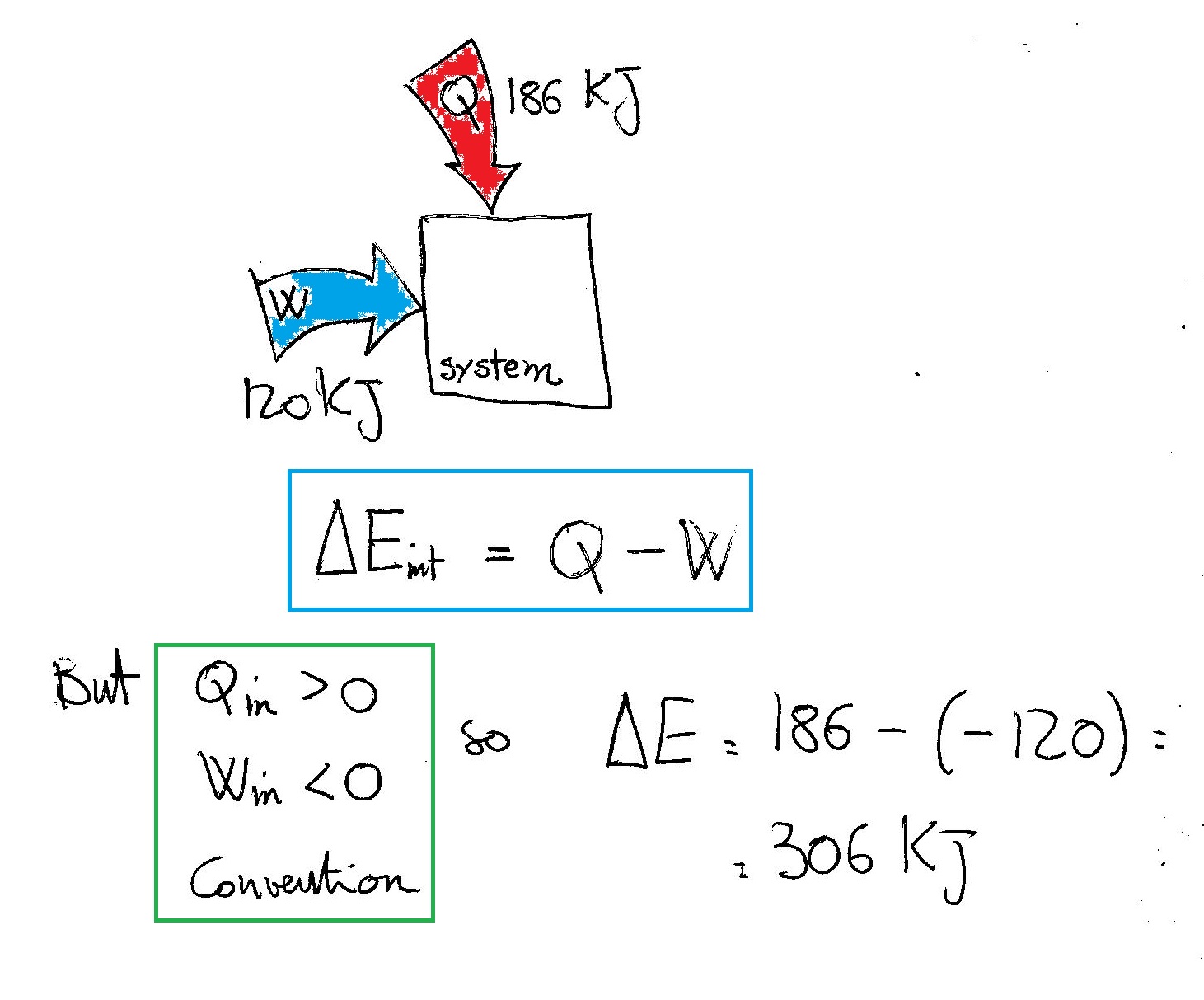
A system absorbs 186 kJ of heat and the surroundings do 120 kJ of

Handbook on Energy Conscious Buildings by Supplementary
AC POWER ANALYSIS Instantaneous & Average Power - ppt download
spectroscopy - A compound that absorbs all visible light
What is the color of an object that absorbs all colors except red
Hampton Bay Welcome Gnome with Solar Lighted Lantern
Zydus Hospitals Hyperglycemia refers to high levels of sugar or
 The Political Effects of the American Civil War
The Political Effects of the American Civil War CELINE VERNEUIL ANKLE BOOT WITH TRIOMPHE IN CALFSKIN - BLACK
CELINE VERNEUIL ANKLE BOOT WITH TRIOMPHE IN CALFSKIN - BLACK Gaecuw Leggings for Women Butt Lift Jeans Slim Fit Scrunch Long
Gaecuw Leggings for Women Butt Lift Jeans Slim Fit Scrunch Long Crest Tank – PRIMARCHÉ
Crest Tank – PRIMARCHÉ Freya Fancies Underwire Molded Balcony Bra, Petal – Bras & Honey USA
Freya Fancies Underwire Molded Balcony Bra, Petal – Bras & Honey USA FENTY BEAUTY Gloss Bomb Cream - Colour Drip Lip Cream
FENTY BEAUTY Gloss Bomb Cream - Colour Drip Lip Cream