Pick only the incorrect statement.for gas A, a=0,the
4.5 (149) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:pick only the incorrect statement
Click here👆to get an answer to your question ✍️ Pick only the incorrect statement-for gas A- a-0-the compressibility factor is linearly dependent on pressure-for gas C-aneq 0-bneq 0-it can be used to calculate a and b by giving lowest P value-for gas B-0-if b-0-the compressibility factor is lineraly dependent on pressure-slope all three gases high pressure is positive
Solution- -C-xA0-for gas C-a-x2260-0-b-x2260-0- it can be used to calculate a and b by giving lowest P value-According to the real gas equation-The constants -apos-a-apos- and -apos-b-apos- are Van der Waals constant for attraction and volume for a given gas-The -apos-a-apos- values for a given gas are measure of intermolecular forces of attraction- More are the intermolecular forces of attraction- more will be the value of a-xA0-For a given gas van der Waals constant of attraction -apos-a-apos- is always greater than van der Waals constant of volume -apos-b-apos-xA0-The gas having higher value of -apos-a-apos-xA0- can be liquefied easily and therefore H2 and He are not liquefied easily-According to this- for gas A-Z-gt-1-a-0 and its dependence on P is linear at all pressure and for gas B-Z-lt-1-b-0 and its dependence on P is linear at all pressure-Also- at high pressure- the slope is positive for all real gases
How to Write Topic Sentences

Solved Select the incorrect statement from the following

Solved Question 13: Select all the correct statement(s); 1
/app/uploads/sites/28/201

How To Select A Turbo Part 2: Calculations - Garrett Motion

Select incorrect statement(s) (a) At very low pressure real gases

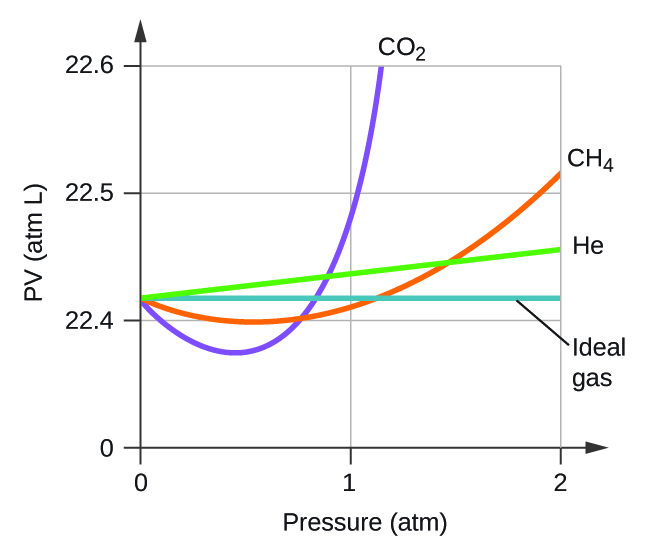
The given graph represents the variation of compressibility factor

a= Van der Waal's constant for pressure correction b= Van der

Which of the following statement is wrong ? For gas A, a= 0 and z
How to Calculate Compression Ratio: 9 Steps (with Pictures)
Solved Using the virial equation of state, calculate the
the compression factor one mole of a vander waals gas 0 C and 100
 Cotton On Jacquard Corset Crop Tank
Cotton On Jacquard Corset Crop Tank Fajas Colombianas Salome La Original Short Lipoescultura Moldeador
Fajas Colombianas Salome La Original Short Lipoescultura Moldeador Lifting Up Bra for Women Hide Back Fat Full Back Coverage Fashion Deep Cup Brasieres Sculpting Uplift Sport Bralette (Color : Beige, Size : 44F) : : Clothing, Shoes & Accessories
Lifting Up Bra for Women Hide Back Fat Full Back Coverage Fashion Deep Cup Brasieres Sculpting Uplift Sport Bralette (Color : Beige, Size : 44F) : : Clothing, Shoes & Accessories Flat Narrow V Wire Bra Separator
Flat Narrow V Wire Bra Separator Yoga Mat Bag Gym Backpack Large Capacity Yoga Bag Luggage Backpack Carrier, Yoga Bag Gym Bag Training Mats
Yoga Mat Bag Gym Backpack Large Capacity Yoga Bag Luggage Backpack Carrier, Yoga Bag Gym Bag Training Mats Womens Plus Size Tops Square Neck Puff Short Sleeve Loose Fit Casual Solid Summer Tee T Shirts (1X-5X)
Womens Plus Size Tops Square Neck Puff Short Sleeve Loose Fit Casual Solid Summer Tee T Shirts (1X-5X)