Solved] Why is the compressibility factor less than 1 at most conditions?
4.6 (633) In stock

SOLUTION: Statistical molecularthermodynamics homework solution2 - Studypool

12 Questions on Physical Chemistry for Biochemists I with Answers, CHEM 340, Exams Chemistry
What is the value of compressibility factor for real gases at critical conditions? - Quora

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is

Determine Compressibility of Gases

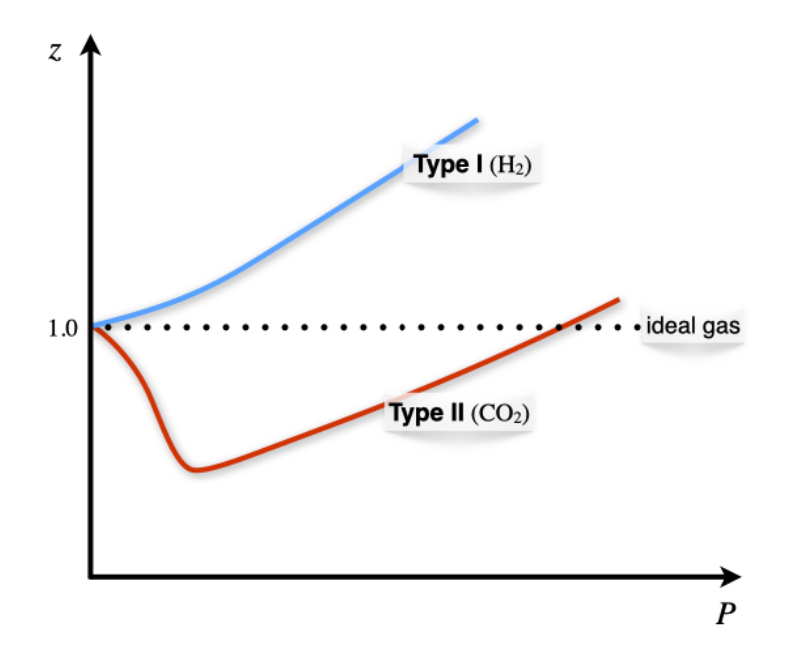
Compressibility factor - Wikipedia

Non-Ideal Gas Behavior Chemistry: Atoms First
11.3: Critical Phenomena - Chemistry LibreTexts

Isothermal Compressibility. - an overview

Vertical & Horizontal Compression of a Function - Lesson

Compressibility factor (z): real gases deviate from ideal behav-Turito
- Tommee Tippee Made for Me Single Electric Wearable Breast Pump - NEW OPEN BOX
 Cable Matters USB C to USB B 3.0 Cable 3.3 ft (USB C to USB Type B 3.0, 3.0 USB B to USB C) in Black
Cable Matters USB C to USB B 3.0 Cable 3.3 ft (USB C to USB Type B 3.0, 3.0 USB B to USB C) in Black Sports Bras Without Removable Pads Sexy Lingerie Bra 36Gg Sports Bra Women Seamless Bra Best Bras For Pregnancy Supportive Pumping Bra Long Line Sports Bra Plus Size Front Closure Bras For Seniors
Sports Bras Without Removable Pads Sexy Lingerie Bra 36Gg Sports Bra Women Seamless Bra Best Bras For Pregnancy Supportive Pumping Bra Long Line Sports Bra Plus Size Front Closure Bras For Seniors Miu Miu Black Velvet Wide Leg Pants Size 40 – Mine & Yours
Miu Miu Black Velvet Wide Leg Pants Size 40 – Mine & Yours Feather Down Comforter King, Beautiful Pinch Pleat Duvet Insert, 100% Cotton Fabric, All Season 106 x 90 in. 8933YW4SSA1 - The Home Depot
Feather Down Comforter King, Beautiful Pinch Pleat Duvet Insert, 100% Cotton Fabric, All Season 106 x 90 in. 8933YW4SSA1 - The Home Depot D.A.N.C.E - Legging, Dance Costumes
D.A.N.C.E - Legging, Dance Costumes