The compressibility factor Z a low-pressure range of all gases
4.8 (776) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor z at a lowpressure range of all gases except hydrogen is
Click here👆to get an answer to your question ✍️ The compressibility factor Z a low-pressure range of all gases except hydrogen is-Z-1- displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-Pb-RT-Z - - 1 - displaystylefrac-Pb-RT-
The van der Waals equation for real gases is -P-aVm2-Vm-x2212-b-RT

PDF) New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms

Compressibility factor - Wikipedia

Solved The graph of compressibility factor (Z)v/sP for 1 mol

Chemistry!!! Not Mystery : Do Real Gases Behave Ideally?

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

Gas compressibility factor Z: Ideal gas vs Real gas

Chemistry Desk: Effect of Pressure

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor
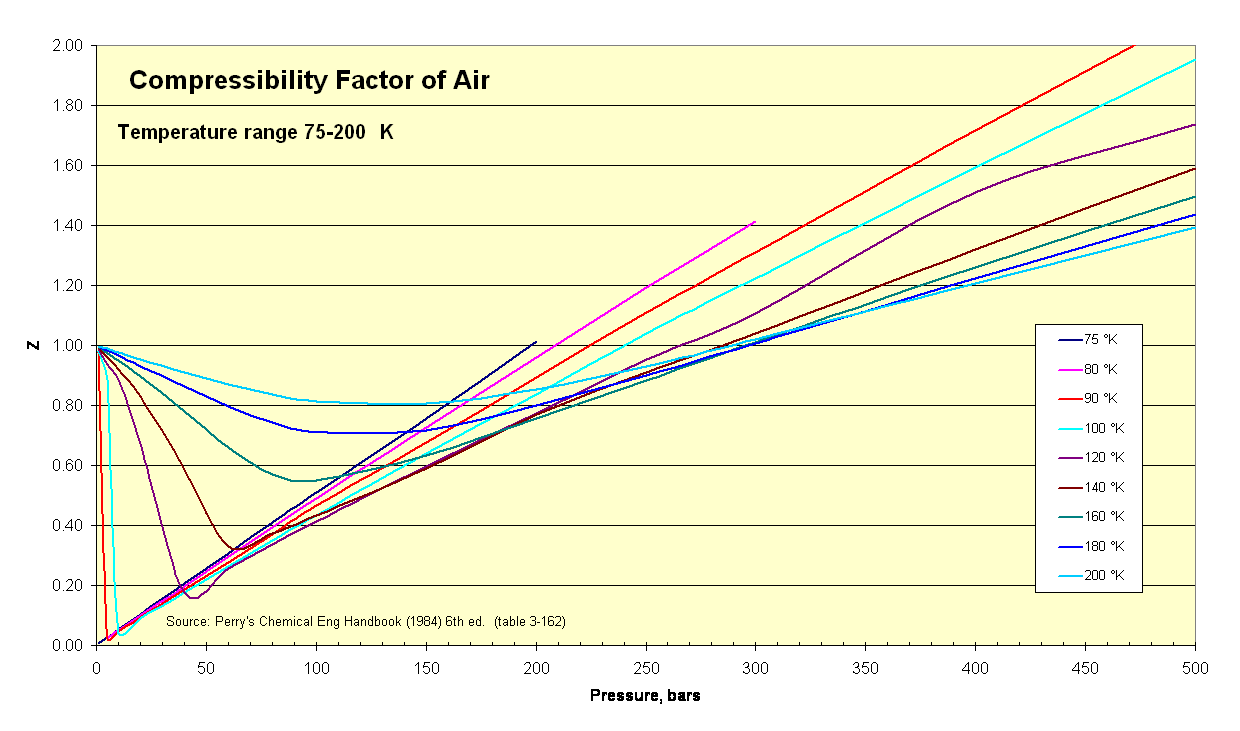
Air Compressibility Factor Table - EnggCyclopedia
Physical Chemistry The Compression Factor (Z) [w/1 example]
Answered: (a)Using the compressibility chart,…
What is the compressibility factor (Z) for 0.02 mole of a van der Waal
In the above figure, near the point B, compressibility factor Z is about..
Consider the graph between compressibility factor Z and pressure P
 Slip in Photo Album for 300 4x6 Photos, Personalised Velvet Photo
Slip in Photo Album for 300 4x6 Photos, Personalised Velvet Photo L/XL Extra Large Compression Calf Sleeve Futuro Sport 3M leg wrap brace NIB
L/XL Extra Large Compression Calf Sleeve Futuro Sport 3M leg wrap brace NIB Box braids: o que é, como fazer, tutoriais e fotos (GUIA)
Box braids: o que é, como fazer, tutoriais e fotos (GUIA)- Passionata By Chantelle Teddie Fishnet Spacer Bra
 40D Bra Size Online Shopping in Pakistan, Buy 40D Bra Size Online
40D Bra Size Online Shopping in Pakistan, Buy 40D Bra Size Online 2x2 formal attire template female #formal #attire #women #2x2
2x2 formal attire template female #formal #attire #women #2x2
