Color change is only device modification. Is a new 510k required? - Medical Device Academy
4.8 (326) In stock

This article explains the process for determining if a color change and other material changes require a new 510k prior to implementing the change.

UDI Procedure (SYS-39) and Webinar Bundle

Medical Device UDI Requirements in the US and Europe

Does My Modified Medical Device Require a New 510(k)?

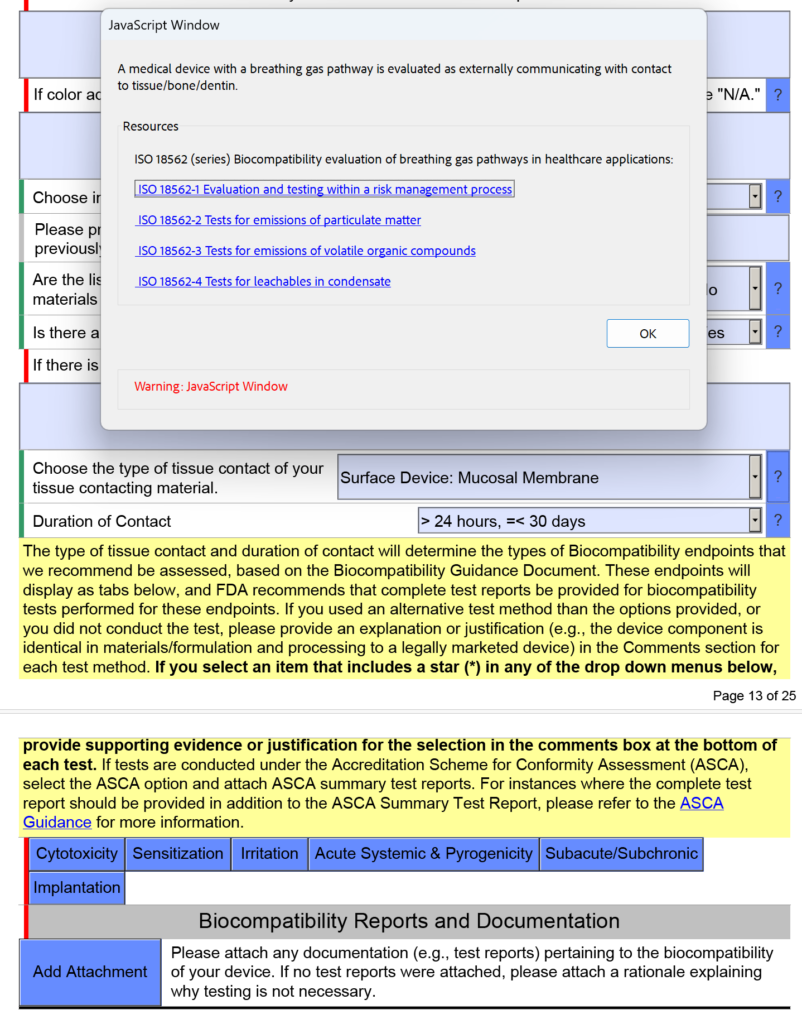
FDA Guidance on 510(k) for Changes to Existing Devices

Case Study: FDA Regulatory Responsibilities for Color Additives

How Long Does a 510(k) Actually Take?

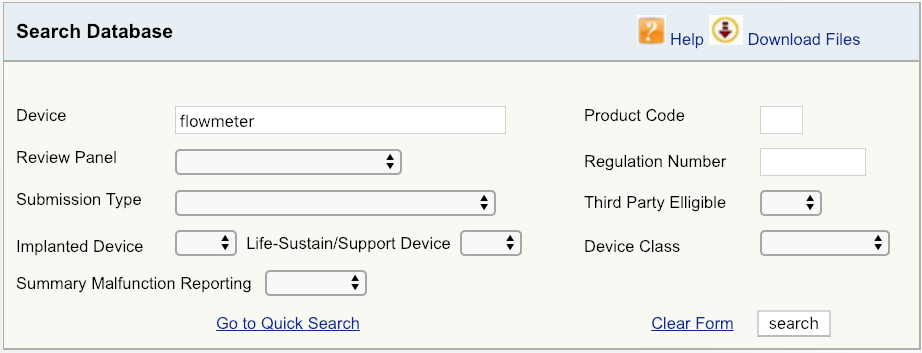
The FDA submission process: 510K vs PMA. What's the difference?
The FDA 510(k) Process: Setting the Stage for a Successful
IFU for Medical Devices, a Definitive Guide (EU & US)
Medical Device Academy Blog Archive
FDA

What is new in the IEC 62366-1 AMD1:2020? - Medical Device HQ
Does Your Device Modification Qualify For A Special 510(k)?
%20submission%20types.png?width=1758&height=570&name=510(k)%20submission%20types.png)
When to submit a 510(k) vs. a Premarket Approval
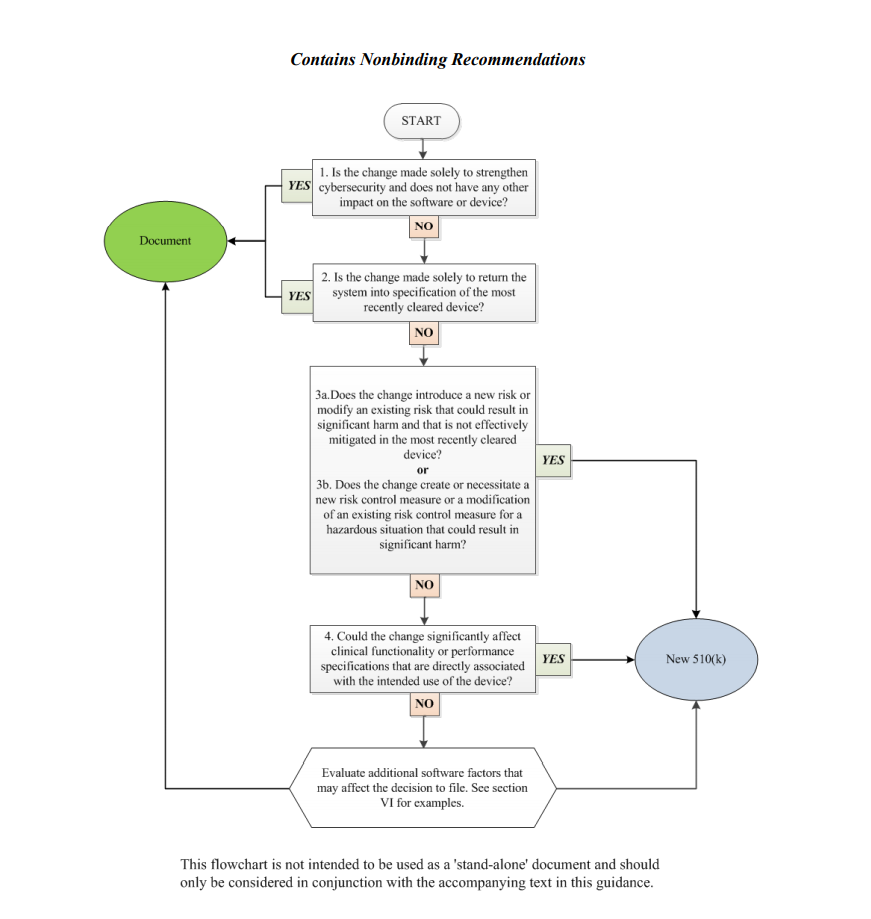
FDA: How to Tell When a Software Change Requires a New 510(K
Color Changing Dual Ended Markers - 8 Count, Crayola.com
Color Change Sapphire - Color First
Jet-Puffed Debuts Color-Changing Marshmallows for S'Mores Season
How to Install Color Changing LEDs in a Room - LEDSupply Blog
 MPL30 Cinch Straps Compression - EVERGOODS
MPL30 Cinch Straps Compression - EVERGOODS- Camisa Mujer + Short Conjunto Manga Larga Informal Art 181
 You can still titty fuck an A. I have small boobs, 32A. At least I don't get back pain and can sleep comfortably
You can still titty fuck an A. I have small boobs, 32A. At least I don't get back pain and can sleep comfortably Nipslip Porn - nipslip & nipslip Videos - SpankBang
Nipslip Porn - nipslip & nipslip Videos - SpankBang Eclat Wide-Leg Brocade Pant
Eclat Wide-Leg Brocade Pant 7 Outfit Ideas to Bring Out Your Best as You Enjoy Pilates
7 Outfit Ideas to Bring Out Your Best as You Enjoy Pilates
