Percentage Yield of a Chemical Reaction. Let's look at your last Chemistry Test You scored 32/40. What's your % grade? (32/40) * 100% = 80% What is the. - ppt download
4.6 (694) In stock

What has this to do with Chemistry? Theoretical yield of a chemical reaction is predicted by stoichiometry. The amount of product obtained by the chemist is the actual yield.
Percentage Yield of a Chemical Reaction
What’s your % grade. (32/40) * 100% = 80% What is the theoretical grade on this test. (theoretical = highest possible grade) 40 What was your actual grade. 32.
Theoretical yield of a chemical reaction is predicted by stoichiometry. The amount of product obtained by the chemist is the actual yield..
Actual yields are often less than theoretical yields due to competing (side) reactions loss of product due to poor lab technique chemical equilibrium (See y’all next year!) impure reactants
Actual yields can also be greater than theoretical yields due to an impure or contaminated product a solid product that hasn’t been sufficiently dried
ie. grams/grams; mol/mol, etc.
in units of grams. 2. Calculate % yield..
Theoretical yield is 1 mol N 2 (g):2 NH 3 (g) 7.5 g ↓(/28.0 g/mol) 0.27 mol (x 2/1) 0.54 mol ↓x 17.0 g/mol 9.1 g NH 3 is the theoretical yield
% =(actual/theoretical) * 100% = (1.7g/9.1g) * 100% =19% (to two sf) Does this answer make sense
Sample Problem 2 Calcium carbonate, CaCO 3, thermally decomposes to produce CaO and CO 2 according to CaCO 3 (s) CaO(s) + CO 2 (g) If the reaction proceeds with a 92.5% yield, what volume, at SATP, of CO 2 can be expected if 12.4 g CaCO 3 is heated
1 mol CaCO 3 (s):1 mol CO 2 (g) 12.4 g ↓/ g.mol mol (x 1/1) mol ↓ x 24.0 L/mol 2.97 L theoretical ↓ x yield 2.75 L actual yield at SATP
p 262 PP 31 – 33 p 264 PP 34 – 37 Homework there’s more
For example, the reactant you massed is only 70% pure. What will this do to the % yield. Yield will be 70%..
When a 13.9 g sample of impure iron pyrite is heated in the presence of oxygen, O 2, 8.02 g of Fe 2 O 3 is produced according to: 4 FeS 2 (s) + 11 O 2 (g) 2 Fe 2 O 3 (s) + 8 SO 2 (g) What is the % purity of the iron pyrite sample .
% purity= (12.0 g/13.9 g) * 100% = 86.3% is the purity of iron pyrite.
Homework PP #38, 39, 40 on p 269 SR #1 – 4 on p 270 Get started on Ch 7 review problems.

Study Guide Percent Yield WS 2 2021, PDF

Calculate Percent Yield with Ideal Stoichiometry - Practice - 1

Quantitative Aspects of Chemical Change: Percentage Yield

How To Calculate Theoretical Yield and Percent Yield

Percentage Yield of a Chemical Reaction. Let's look at your last

How To Calculate The Percent Yield and Theoretical Yield

Percent Yield - Video Tutorials & Practice Problems
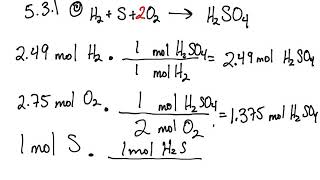
5.3: Calculating Reaction Yields (Problems) - Chemistry LibreTexts

Percent Yield in a Chemical Reaction (lab) by Science and The Big

Percentage Yield of a Chemical Reaction. Let's look at your last
Higher temps mean higher food and other prices. Study links climate shocks to inflation
Budget Padding Allegation: Senator Adeola Reads Transcription Of Senator Ningi's Interview
Railway ALP Exam 2024 Free Online Classes, Previous Years Question Series
 Best Deal for Cebbay Sorry This Booty is Taken by A Crazy
Best Deal for Cebbay Sorry This Booty is Taken by A Crazy Casey Kevin Jockstraps For Men Jock Strap Wide Waistband Athletic Supporters Sexy Underwear, CK2213-Black, Medium : : Clothing, Shoes & Accessories
Casey Kevin Jockstraps For Men Jock Strap Wide Waistband Athletic Supporters Sexy Underwear, CK2213-Black, Medium : : Clothing, Shoes & Accessories Waist training 101: Waist Trainers vs. Corsets (What's the Difference?) - Hourglass Angel
Waist training 101: Waist Trainers vs. Corsets (What's the Difference?) - Hourglass Angel Bloch Ladies Kalise Capri Leggings - Bath Dancewear
Bloch Ladies Kalise Capri Leggings - Bath Dancewear CLOSE-OUT SALE-ALWAYS Discreet Women's Underwear 19ct Small/Medium
CLOSE-OUT SALE-ALWAYS Discreet Women's Underwear 19ct Small/Medium Gray cloth fabric texture to download - ManyTextures
Gray cloth fabric texture to download - ManyTextures