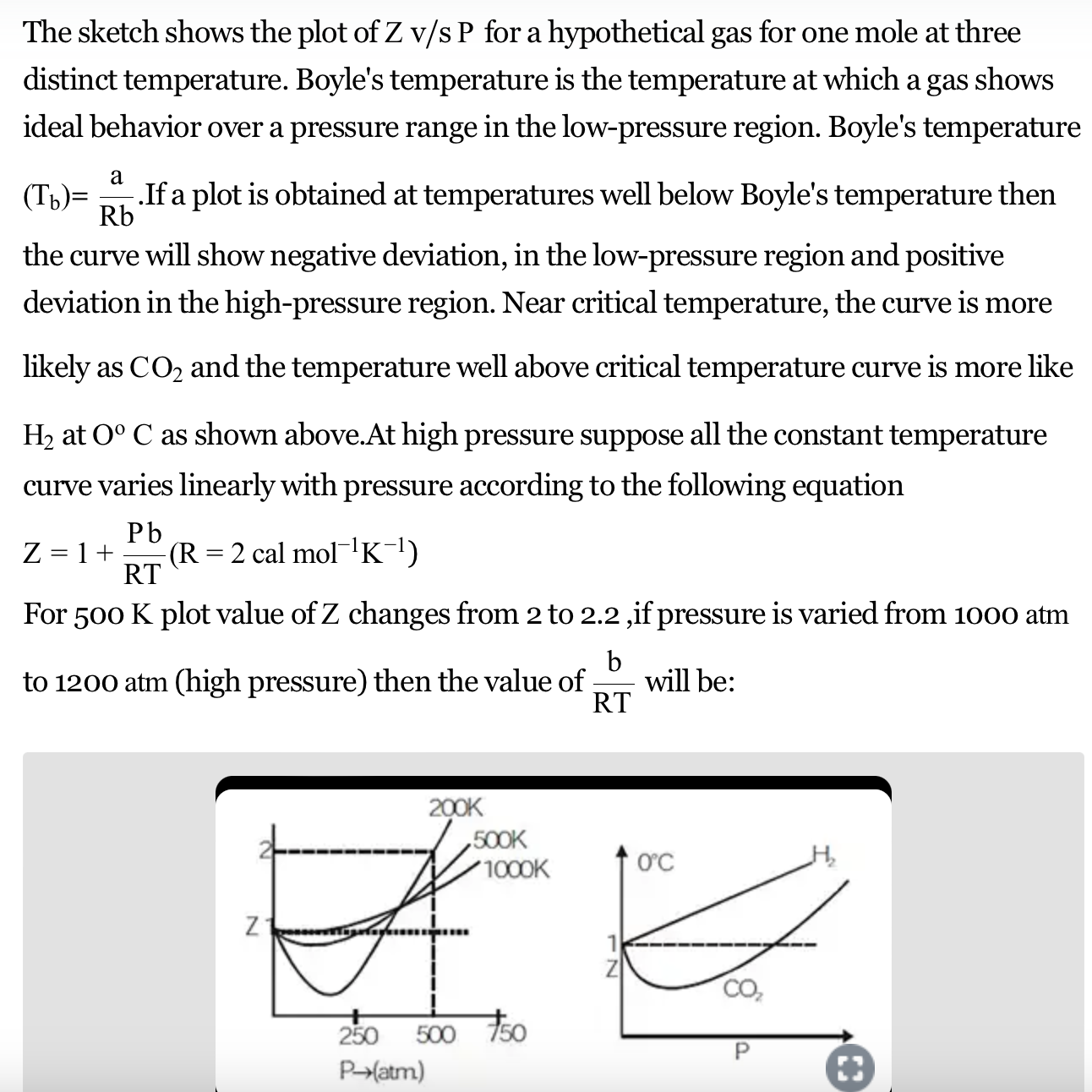
What is the compressibility factor? What is its value an ideal gas
4.5 (199) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:what is the compressibility factor what is its value for an ideal gas how does
Click here👆to get an answer to your question ✍️ What is the compressibility factor- What is its value an ideal gas- How does it to understand the extent of deviation of a gas from ideal behavior
Ideal gases and real gases are compressible or not compressible what is the compressible factor for real gases and ideal gases.

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Compressibility Factor - an overview

For an ideal gas, the value of compressibility factor is zero.

Real Gases Introductory Chemistry

Compressibility Factor Calculator - File Exchange - MATLAB Central
Solved The graph of compressibility factor (Z)v/sP for 1 mol

Non-Ideal Gas Behavior Chemistry: Atoms First

Solved The definition of compressibility factor Z, Eq.

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z=(1-displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
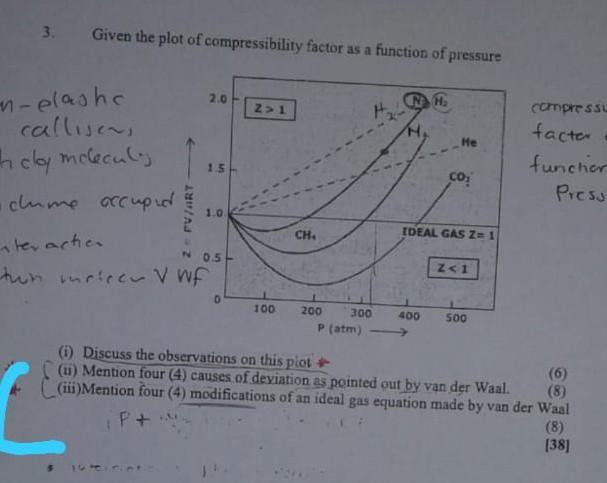
Solved 3. Given the plot of compressibility factor as a
Compressibility factor (gases) - Knowino
Solved Using the chart, the compressibility factor (Z), for
Solved The plot below shows how compressibility factor (Z)
thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange
 Microfiber Sand Free Beach Towel Thin Quick Fast Dry Super Absorbent Oversized Large Lightweight Towels For Travel Sports Pool Swimming Bath Camping Yoga Girls Women Adults Boho Palm Tree Blue
Microfiber Sand Free Beach Towel Thin Quick Fast Dry Super Absorbent Oversized Large Lightweight Towels For Travel Sports Pool Swimming Bath Camping Yoga Girls Women Adults Boho Palm Tree Blue Lily Loves Cut Out Ribbed One Piece Swim Bathers
Lily Loves Cut Out Ribbed One Piece Swim Bathers Women's Tshirt Dress Pockets Floral Cover Ups Short Sleeve Loose Flowy Sundresses (Black,S) at Women's Clothing store
Women's Tshirt Dress Pockets Floral Cover Ups Short Sleeve Loose Flowy Sundresses (Black,S) at Women's Clothing store Pacific & Park Core Twill Slim Fit Jogger Pants Black-Size Small 30-32W
Pacific & Park Core Twill Slim Fit Jogger Pants Black-Size Small 30-32W Nike Sportswear Tech Fleece Men's 1/2-Zip Sweatshirt
Nike Sportswear Tech Fleece Men's 1/2-Zip Sweatshirt- Cotton High-Leg Brief Panty 15B 112
