What is the compressibility factor (Z) for 0.02 mole of a van der
4.7 (768) In stock


Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download

its mole fraction. Solution : P=KH⋅X⇒PCO2( g)=KH⋅X(CO2)⇒0.01=35×100..

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

Problem Set 2 Solutions

Physical Chemistry The Compression Factor (Z) [w/1 example]

compressible flow related terms - Department of Mechanical and

SOLUTION: M2ex flat plate - Studypool
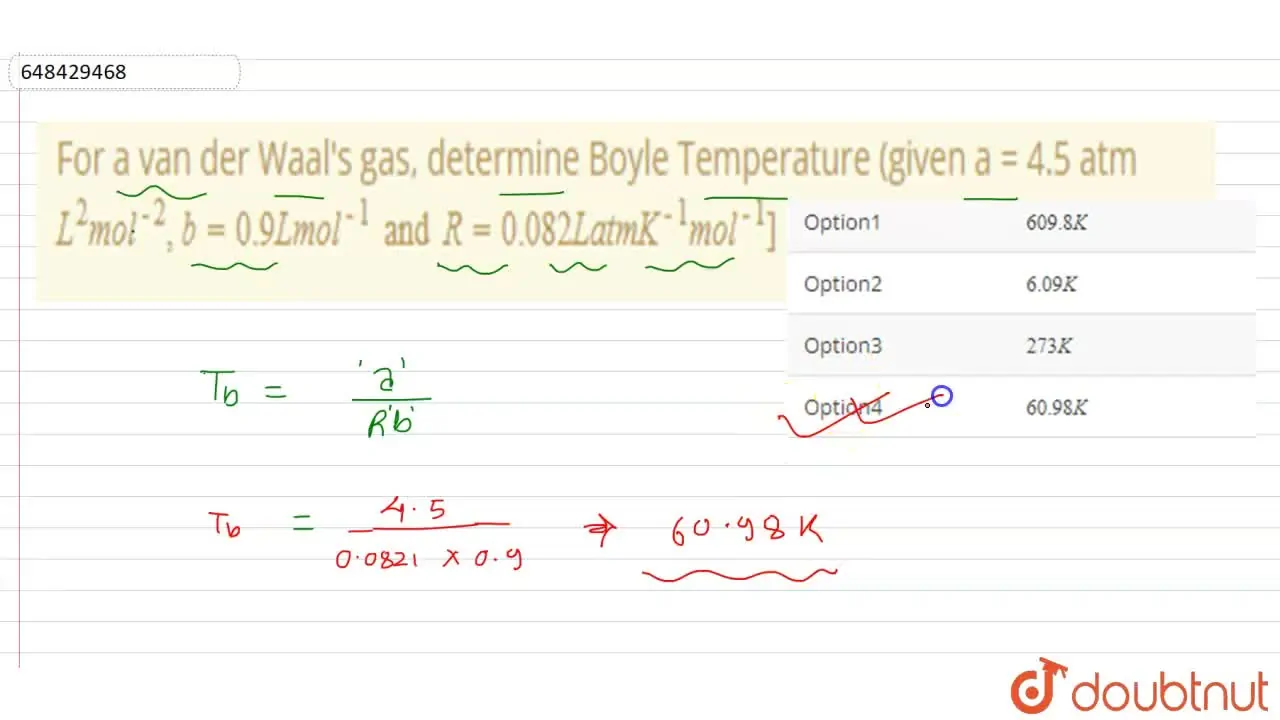
For a van der Waal's gas, determine Boyle Temperature (given a = 4.5 a

PDF) New Correlation for Hydrogen-Natural Gas Mixture Compressibility Factor

Solved Please answer all the questions and explain how the
Compressibility Factor Calculator
Solved F The compressibility factor ( Z ) of liquid faca
3.2 Real gas and compressibility factor – Introduction to
 Pasta Amendoim PowerOne Crocante 1.005kg
Pasta Amendoim PowerOne Crocante 1.005kg Push up Bikini Tops for Women Small Bust Women Plus Size Floral
Push up Bikini Tops for Women Small Bust Women Plus Size Floral Babydoll Lacy Net V-Neck Dress - Red
Babydoll Lacy Net V-Neck Dress - Red Barbiecore: 22 Barbie Fashion Finds to Buy in 2023 - Shop Barbie Fashion
Barbiecore: 22 Barbie Fashion Finds to Buy in 2023 - Shop Barbie Fashion- Tender Care - Give your little one hypo-allergenic care
 Women Autumn Casual Midi Dress Chiffon Red Long Sleeve Blue Maxi Dresses, Black, Small : : Clothing, Shoes & Accessories
Women Autumn Casual Midi Dress Chiffon Red Long Sleeve Blue Maxi Dresses, Black, Small : : Clothing, Shoes & Accessories
