What is the compressibility factor (Z) for 0.02 mole of a van der Waal
4.5 (367) In stock

(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

ecreases (C) remains same (D) changes unpredictably 16.a What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules

If Z is a compressibility factor, van der Waals equation at low
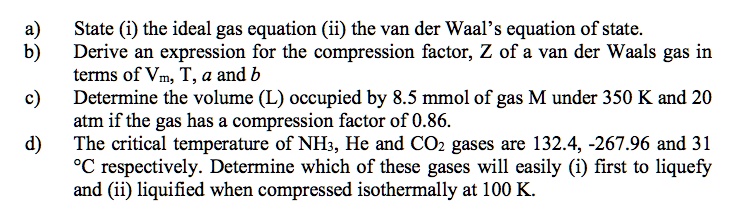
SOLVED: State (i) the ideal gas equation (ii) the van der Waal's

Poulduly 59. What is the compressibility fac is the compressibility factor (Z) 0.02 mole co Vanderwaals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. . RT =

Punjabi] What is the compressibility factor (Z) for 0.02 mole of a va

20 dm^(3) of SO(2) diffuse through a porous partitions in 60 second Wh

What is the compressibility factor (Z) for 0.02 mole of a van der

What is the compressibility factor (Z) for 0.02 mole of a van der

Compressibility Factor Calculator - File Exchange - MATLAB Central
Physical Chemistry The Compression Factor (Z) [w/1 example
Compressibility Factor (Z) And Pressure Bar Royalty Free SVG
physical chemistry - Why do some gases have lower value of Z for a
Table 2 from Compressibility Factor of Gas with High Content of
The graph of compressibility factor (Z) vs. P for one mole of a real gas ..
 pantalones vaqueros
pantalones vaqueros Mamalicious Maternity Maternity Clothes - Matalan
Mamalicious Maternity Maternity Clothes - Matalan Under Armour Girls Fly By Shorts Black XL
Under Armour Girls Fly By Shorts Black XL parka tri-mountain azul s - Buena Ropa
parka tri-mountain azul s - Buena Ropa Hippie Bell Bottom Jeans OOAK Custom Order Send Me YOUR Jeans Unique Flare Unisex Free Plus Size Men Women Halloween Costume Bell Bottoms - Canada
Hippie Bell Bottom Jeans OOAK Custom Order Send Me YOUR Jeans Unique Flare Unisex Free Plus Size Men Women Halloween Costume Bell Bottoms - Canada Unisex Black Metal Spike Studded Punk Rock Biker Wide Strap Leather Bracelet Chain Wristband
Unisex Black Metal Spike Studded Punk Rock Biker Wide Strap Leather Bracelet Chain Wristband