Solved] Why is the compressibility factor less than 1 at most
5 (469) In stock
Answer to Why is the compressibility factor less than 1 at most conditions?

Compressibility factor - Wikipedia

Compressibility factor (z): real gases deviate from ideal behav-Turito

Statement-1 is correct, Statement-2 is incorrect.

SOLUTION: Statistical molecularthermodynamics homework solution2

Van der Waals Equation - Derivation, Relation Between Ideal Gas

Acentric Factor - an overview

gas laws - Compressible Factor - Chemistry Stack Exchange
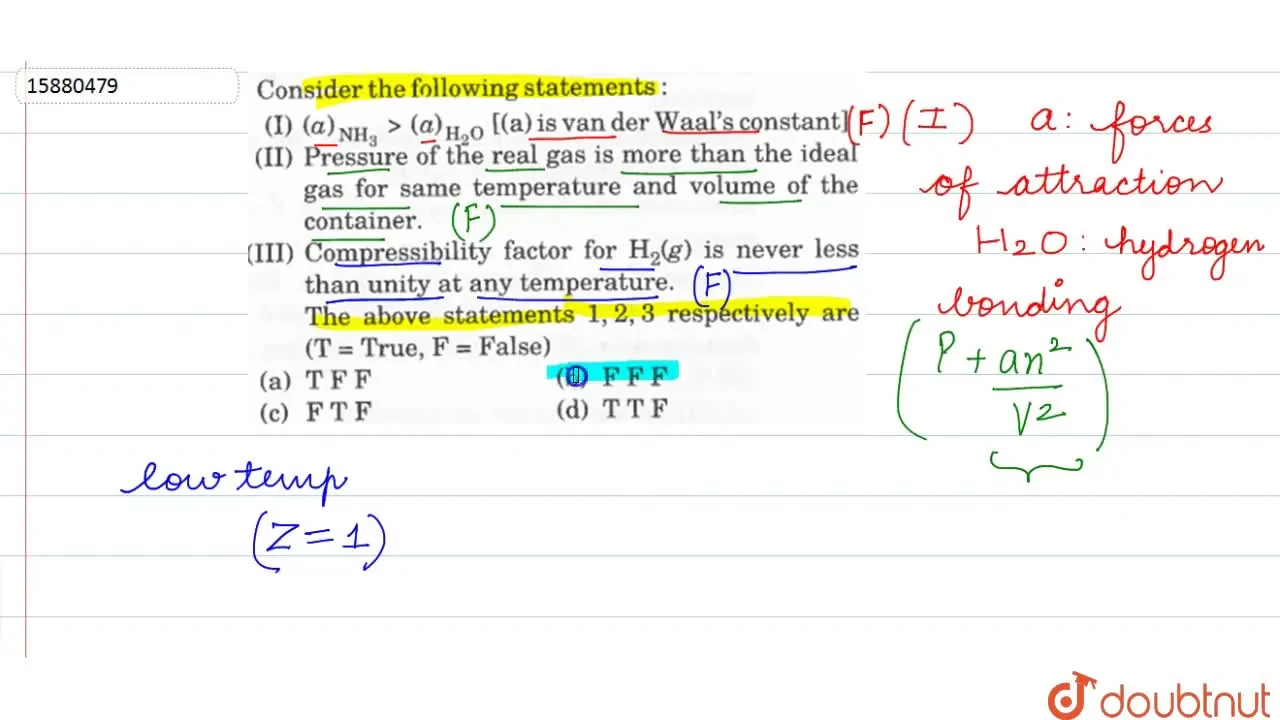
Consider the following statements: (I) (a)(NH(3))gt(a)+(H(2)O) [(a)

Except H(2) and He, the compressibility factor Z(=(PV)/(nRT))lt1 for a
Excel Calculations: Compressibility Factor Calculator for Excel
Slope of graph of compressibility factor(Z) with pressure(P) for hydrogen gas at any pressure i
a) A gas at 250 K and 15 atm has a molar volume 12 per cent
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT
 NWT- Lululemon Define Jacket Luon- Strawberry India
NWT- Lululemon Define Jacket Luon- Strawberry India Elomi Essentials Bandeau Bikini Black ES7532 – Bras & Honey USA
Elomi Essentials Bandeau Bikini Black ES7532 – Bras & Honey USA Sari Petticoat Stitched Indian Saree Petticoat
Sari Petticoat Stitched Indian Saree Petticoat Saree Blouses Round Deep Neckline 30″ - Pattern Faculty Com
Saree Blouses Round Deep Neckline 30″ - Pattern Faculty Com Vintage Rock Double Collar Studded Sleeveless Twofer Dress in
Vintage Rock Double Collar Studded Sleeveless Twofer Dress in Women Sexy Panties,lace Thongs G-string With Pearls Ball Ladies
Women Sexy Panties,lace Thongs G-string With Pearls Ball Ladies