physical chemistry - Is the compressibility factor smaller or
4.8 (448) In stock

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Compressibility Factor (Z-Factor) Equation of State

Physical Chemistry The Compression Factor (Z) [w/1 example

What Exactly is The Compressibility of Fluids?

Van der Waals Equation - Derivation, Relation Between Ideal Gas

Physical Chemistry The Compression Factor (Z) [w/1 example

Explain how the compression factor varies with pressure and

The compressibility factor `(Z)` of real gas is usually less than
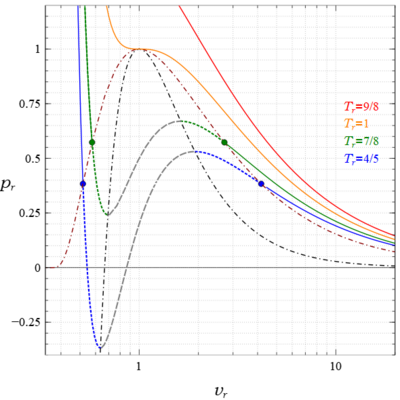
upload.wikimedia.org/wikipedia/commons/thumb/6/6e/

Physical Chemistry The Compression Factor (Z) [w/1 example

Inorganic and Physical Chemistry Testbank 2022, PDF

Compressibility factor - Wikipedia
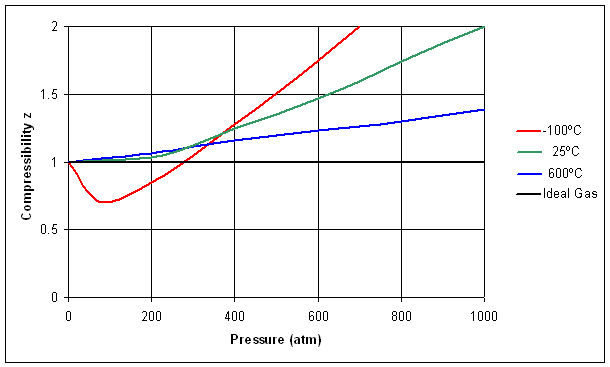
Gas Laws – First Year General Chemistry
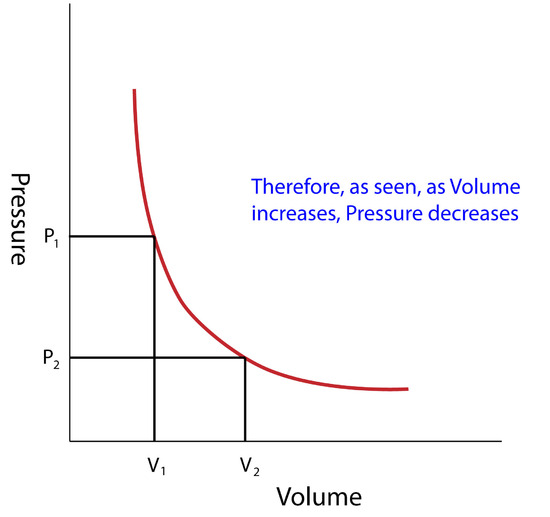
Gas Laws - Overview - Chemistry LibreTexts

Compressibility factor (gases) - Knowino
COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!
Generalized Compressibility Chart - Dr. Javier Ortega Pages 1-31
Compressibility Factor (Z) and pressure bar Stock Vector Image & Art - Alamy
 Leggings Depot Women's Maternity Leggings Over The Belly Pregnancy Casual Yoga Tights(Full Length Cherish Rose) - Leggings Depot
Leggings Depot Women's Maternity Leggings Over The Belly Pregnancy Casual Yoga Tights(Full Length Cherish Rose) - Leggings Depot Underscore Shapewear - UK
Underscore Shapewear - UK ZMPSIISA Women High Waisted Cargo Pants Wide Leg
ZMPSIISA Women High Waisted Cargo Pants Wide Leg Vintage 1950s Girdle and Bra Sewing Pattern | Vintage Shapewear Sewing Pattern
Vintage 1950s Girdle and Bra Sewing Pattern | Vintage Shapewear Sewing Pattern Buy Yoga Bar 100% Peanut Butter - Creamy, Roasted, High In
Buy Yoga Bar 100% Peanut Butter - Creamy, Roasted, High In Brown Sculpted Beaver Fur Jacket Fox Fur Collar and Cuffs
Brown Sculpted Beaver Fur Jacket Fox Fur Collar and Cuffs